the walls of the aortic vestibule, which is the septo-aortic and mitral-aortic continuity.1 For more details on cardiac embryogenesis, we recommend monographs and review articles on this subject.1, 2, 3, 4
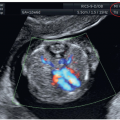
can be obtained in the first trimester under optimal scanning conditions. Based upon our experience however, visualization in the first trimester of four essential planes—(1) the axial view of the upper abdomen, (2) the 4CV in gray scale, (3) the 4CV in color Doppler, and (4) the 3VT in color Doppler (Fig. 11.5)—provides enough information to rule out most major cardiac malformations.
Table 11.1 • Optimization of the gray-scale cardiac examination in the first trimester | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Table 11.2 • Optimization of Color Doppler Cardiac Examination in the First Trimester | ||||||||
|---|---|---|---|---|---|---|---|---|
|
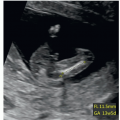
in early gestation. Figure 11.6 shows a fetus at 13 weeks of gestation with deletion 22q11.2 detected with targeted FISH performed due to the presence of polydactyly and an interrupted aortic arch seen on the first trimester ultrasound.
Table 11.3 • Suggested Indications for Fetal Cardiac Imaging in the First Trimester | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
an absent or reversed A-wave during the atrial contraction phase of diastole (Fig. 11.8A). This flow pattern in the first trimester has been associated with an increased risk of aneuploidy. In chromosomally normal fetuses, abnormal DV waveforms have also been shown to be associated with structural cardiac anomalies.17 The underlying pathophysiologic mechanism linking the reversed DV A-wave to fetal CHD is unclear, but an increased right atrial preload as a result of an increase in volume, pressure, or both in CHD could be one of the underlying mechanisms. Detecting a reversed A-wave in the DV increases the risk for the presence of CHD in the fetus.16
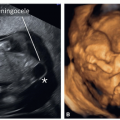
reverse flow on color Doppler (Figs. 11.11C and 11.12C). An echogenic globular left ventricle can occasionally be seen in the first trimester in HLHS (Fig. 11.13) and represents left ventricular changes (fibroelastosis), similar to that noted in the second and third trimesters of pregnancy. Of note is that the presence of a “normal” four-chamber view in the first trimester cannot rule out HLHS, as it has been shown to develop between the first and second trimesters of gestation.
Table 11.4 • Abnormal Ultrasound Findings and Suspected Cardiac Anomalies in the First Trimester | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
associated genetic syndromes, such as Turner syndrome, Noonan syndrome, Smith-Lemli-Opitz syndrome, and Holt-Oram syndrome.21 Fetuses with HLHS may develop growth restriction in the late second and third trimesters of pregnancy probably due to a 20% reduction in combined cardiac output.22 When HLHS is diagnosed in the first trimester, follow-up ultrasound examinations are recommended.
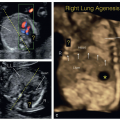
 Figure 11.12: Hypoplastic left heart syndrome in a fetus at 13 weeks of gestation demonstrated by transvaginal ultrasound (different fetus than in Fig. 11.11). Note in A the absence of a left ventricle (LV) in the four-chamber view. In B, color Doppler shows diastolic flow between right atrium (RA) and right ventricle (RV) with absent left ventricular flow. In C, three-vessel-trachea view in color Doppler shows antegrade flow in the pulmonary artery (PA) and retrograde flow into the small aortic arch (AoA). Note the increased resolution in the ultrasound images as compared to Figure 11.11 obtained transabdominally. Compare with Figure 11.11. LA, left atrium. |
 Figure 11.13: Four-chamber view in a fetus with hypoplastic left heart syndrome (HLHS) at 13 weeks of gestation with gray-scale (A) and color Doppler (B) imaging. Note the presence in A of a relatively small echogenic left ventricular (LV) cavity. Color Doppler in B shows absence of mitral inflow during diastole. The presence of an echogenic LV is unusually found in HLHS in the first trimester in comparison with the second trimester. RA, right atrium; RV, right ventricle; LA, left atrium.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|