Author
Tumor model
Endpoint
Results
Additional observations
Blackwood and Cooper [28]
Myosarcoma and carcinosarcoma in rats
Suppression of second tumors and resistance to rechallenge
Significant impact of cryosurgery on regression of 2nd tumors and resistance to rechallenge
Immune response improved with lower volume of residual frozen tissue
Neel et al. [29]
Viral induced mammary adenocarcinoma in C3H/HeN and sarcoma in CDF
Resistance to rechallenge
Superior protection with cryosurgery compared to surgery
Bagley et al. [30]
MCA-10 fibrosarcoma in C57BL6 mice
Cytotoxicity assays of splenic lymphocytes
Tumor-specific lymphocyte mediated cytotoxicity after cryosurgery
Javadpour et al. [31]
Intradermal tumors in guinea pigs
Eradiation of microscopic lymph node metastases
No effect of cryo
Misao et al. [32]
MRMT-1 breast in sprague dawley rats
Resistance to rechallenge
Superior protection at 10 weeks compared to surgery (80 % vs. 18 %, p < 0.001)
Yamashita et al. [33]
KMT-17 fibrosarcoma in WKA/Hok rats
Metastases
Decreased metastases with surgery (11.8 %) compared to cryoablation (48.4 %)
Hayakawa et al. [34]
3MC-induced tumors in WKA/Hok rats
Re-challenge with autochtonous tumor
Greater resistance with surgery (58 %) than cryo (15 %) at 21 days
Tumor growth after rechallenge may have been enhanced
Muller et al. [35]
Dunn sarcoma in the leg of C3H mice
Lung metastases
Decreased lung metastasis with cryo compared to amputation or local resection
No evidence of increased immune response after cryosurgery, possible immune suppression
Wing et al. [36]
HSV-2 fibrosarcoma in hamsters
Cellular responses in the spleen
Increased suppressor T-cells after cryo
Fibrosarcoma in WKA rats
Suppression of contralateral tumors and pulmonary metastases
Suppression of contralateral tumors but enhancement of early pulmonary metastases
Decrease in antitumor T-cell activity with cryosurgery, possibly due to regulatory T-cells
Gazzaniga et al. [22]
Human IIB-JEL-J melanoma in nude mice
Sera antibody response to melanoma antigens
Significant increase in humoral response of cryo compared with untreated
Early peritumoral PMN infiltrate followed by peritumoral macrophages, peaking at day 7
Hoffmann et al. [39]
AT-1 prostate in Copenhagen rats
Resistance to rechallenge
No protection against 2° tumors with cryosurgery
Cryo led to increased anti-tumor antibodies compared to controls, but not to compared with surgical excision
Urano et al. [12]
Colon-26 CA in BALB/c mice
Liver metastases compared to untreated mice
Significant decrease in liver mets after ablation of a single lesion
Joosten et al. [40]
Colon26 tumors in BALB/c mice
Suppression of contralateral tumors
Significant inhibition of secondary tumor growth with cryo
Inhibition correlated with high plasma levels of TNF-a and IL-1a
den Brok et al. [41]
B16-OVA melanoma in C57BL6/J mice
Resistance to rechallenge compared to naïve mice
Moderate level of protection with cryo (50 % vs. 0 %, p < 0.005)
Increased antigen uptake by DC after cryoablation, increased presence of IFN-g producing tumor specific T-cells
Udagawa et al. [42]
CT26 colon CA in BALB/c mice
Suppression of contralateral tumors
No suppression with cryo alone
Sabel et al. [43]
MT-901 mammary adenocarcinoma in BALB/c mice
Resistance to rechallenge
Significant tumor-specific protection after cryoablation
Increased tumor-specific T-cell activation in regional lymph nodes and increased NK function after cryoablation
Machlenkin et al. [44]
Lewis lung carcinoma in C57BL6 mice
Suppression of lung metastases
No change in lung metastases with cryo alone
Redondo et al. [45]
B16/OVA melanoma in
C57BL6/J Mice
Resistance to rechallenge compared to surgery
Low level of protection with cryo (25 % vs. 0 %, p < 0.0001)
Intense infiltrative PMN response to cryo at day 7
Sabel et al. [46]
4T1 mammary carcinoma in BALB/C mice
Pulmonary metastases compared to surgery
Decreased lung metastases and improved survival with cryo
Increased IFN-γ producing T-cells and decreased regulatory T-cells after cryo
Matin et al. [47]
Renca in BALB/c mice
Immune cell infiltration after treatment
Inflammatory infiltrate and cytokine response induced by cryo
Li et al. [48]
C6 gliomas in wistar rats
Peripheral T-cell populations
Increase in CD3+ and CD4+ T-cells
5.3 Anecdotal Evidence of Immune Response to Cryoablation
Among 80 cases of prostate cancer treated cryoablation by Ablin and colleagues, there were several cases where metastatic tumor regressed. As an example, a 68 year old male with bone metastases had two cryoablations, 30 days apart, to relieve obstruction of the primary tumor. Within 2 weeks, the symptoms secondary to the bone metastases resolved and x-rays showed resolution of the lesions. Several other patients had regression of pulmonary, lymph node or bone metastases [21]. All of the patients underwent two in situ ablations at an interval of over 30 days. While it is not absolutely clear that the regression of the metastases was immune-mediated, at least one of the patients had anti-prostatic antibodies detected in their serum after cryosurgery , suggesting a humoral-based response [49]. In another small clinical trial of prostate cryoablation by Horan, there was distal tumor regression in one patient and marked relief of bone pain in others [50].
This observed effect was not limited to cases of prostate cancer. Tanaka treated 49 patients with advanced or recurrent breast cancer with cryosurgery , reporting not only alleviation of pain, control of hemorrhage, and reduction of tumor bulk, but also a 5-year survival of 44.4 % in this group of “incurable” patients [26]. Suzuki described eight patients with stage IV breast cancer who had advanced primary tumors treated by cryosurgery [24]. Two of the eight patients had resolution of distant disease, including regional adenopathy, contralateral metastases and pleural effusions. Uhlschmid et al. [51] described the results of 30 patients who underwent cryoablation of pulmonary metastases. In four patients, regression of contralateral metastases occurred. In one case of malignant teratoma, after ablation of the largest metastases, there was total regression of both the treated lesion and contralateral metastases. A patient with renal cancer had regression of both lung metastases and nodal metastases after cryoablation of several metastatic foci in the left lung. All 4 patients described survived at least 36–48 months after treatment. There were also nine cases where untreated lesions remained stable but did not regress. In three of these patients the untreated lesions ultimately progressed after intervals of 6 and 12 months. Tanaka reported on 307 cases of cryosurgery performed at Hokushin General Hospital in Japan between 1968 and 1981 for which follow-up information was available[25]. Two cases of cryosurgery alone (most of the patients were treated by cryosurgery plus chemotherapy, making it impossible to determine whether distant effects were immunologic) showed evidence of a possible cryoimmunologic effect. A 62 year old male with advanced thyroid cancer had cervical and supraclavicular metastases resulting in edema of the face and a Horner’s syndrome. A partial cryoablation of the thyroid was performed for palliative purposes, followed by the unexpected resolution of the regional metastases, edema and Horner’s syndrome. A 76 year old female with a stage III melanoma of the palate had cryoablation of the primary with what appeared to be immune-mediated eradication of multiple regional metastases.
As these reports came out, subsequent investigators attempted to document this more carefully. Unfortunately, it proved very difficult to demonstrate any consistency to the response. Based on these observations, Ablin et al. [52] suggested that cryosurgery may have more than one immunological effect; in some cases augmenting a tumoricidal effect through cytotoxic T-cells or tumor specific antibodies, while in other cases augmenting a tumor enhancing (immunosuppressive) effect through suppressor T-cells or blocking antibodies.
5.4 How Does Cryoablation Kill Tumors?
Percutaneous cryoablation is performed by inserting one or more cryoprobes in or around the targeted malignant tissue under image guidance. Once in place, the cryoprobe(s) are rapidly cooled, removing heat from the tissue by conduction via physical contact with the cryoprobe. After the cryoablation is completed, the lesion can be allowed to thaw passively or can be actively thawed by heating the cryoprobe. This constitutes one freeze-thaw cycle. Most clinical applications of cryoablation utilize two freeze-thaw cycles for ablation. Cryoablation causes cellular damage through two mechanisms; direct cellular injury, which are the cold-induced injury to the cells, and indirect cellular injury, which are caused by changes to the cellular microenvironment [53, 54] (Figs. 5.1, 5.2and5.3).
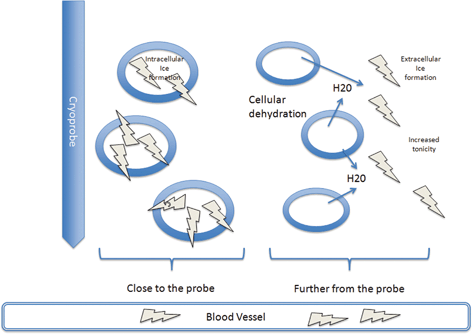
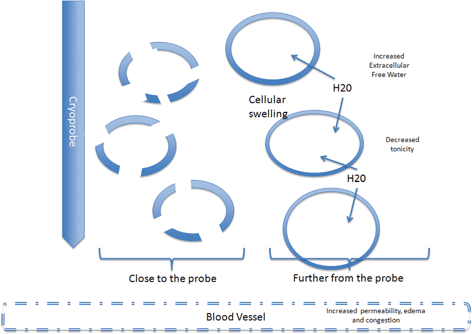

Fig. 5.1
Close to the probe, where freezing is rapid, free water is trapped in the cells and intracellular ice forms. Further from the probe, where freezing is slower, ice forms in the extracellular fluid increasing tonicity and drawing water out from the cells. This damages the enzymes and destabilizes the membrane. Ice also forms within the blood vessels causing damage to the endothelial cells

Fig. 5.2
During thawing the cells close to the probe have undergone necrosis by the intracellular ice. Further from the probe the melting ice will cause increased extracellular free water decreased tonicity and flow of water into the cells. In some cases the cells will burst and necrosis. Others will survive but be irreversibly damaged and die by apoptosis. The damage to the endothelial cell junctions lead to increased capillary permeability

Fig. 5.3
After treatment many cells particularly those close to the probe are necrotic. At the periphery apoptotic cells are cleared by inflammatory cells. Some apoptotic cells will necrose prior to clearance. Reperfusion will bring platelets that contact the damaged endothelial cells causing thrombosis and ischemia. Cells that survive the freezing process will necrose from lack of blood supply
5.4.1 Direct Cellular Injury
As the targeted tissue cools, ice crystals form in the extracellular space. As these ice crystals sequester free water, the tonicity of the extracellular space increases. The resulting osmotic tension draws free intracellular water from the cells, dehydrating them, damaging the cytoplasmic enzymes and destabilizing the cell membrane [55]. However, the impact of intracellular dehydration is dependent upon the speed by which cooling occurs. When cooling is rapid, there isn’t time for these osmotic shifts, and the free water within the cells is trapped, resulting in intracellular ice crystal formation [56]. Although the exact mechanisms are not clear, the mechanism by which intracellular ice formation kills cells is likely due to physical damage to the organelle membranes and the plasma membrane [57]. In a study of patients undergoing hepatic cryoablation, Chapman et al. [58] performed electron microscopy of liver tissue treated by cryoablation and found a disruption of the plasma membrane with extension of intact hepatocyte organelles into the space of Disse, a process called disruptive necrosis. This is different from the coagulative necrosis of the hepatic organelles within an intact plasma membrane that is seen with heat-based ablation, such as radiofrequency ablation (RFA) .
It is important to note that much of the direct cellular injury comes from the thawing of the tissue. Melting ice within the extracellular space now causes hypotonicity compared to the intracellular compartment. Now osmotic forces reverse, leading to swelling and possible bursting of cells. The influx of free water into the intracellular space can increase the growth of intracellular ice crystals, magnifying their effect. This is because the high extracellular solute concentrations lower the freezing point of water [53, 59]. During thawing, intracellular ice formation is maximized at - 20 to - 25 °C [60].
The physical effects of the intracellular ice formation primarily lead to necrosis of the cells. However, not all of the cells are immediately killed by direct cryoablation-induced necrosis, particularly those cells that are at the periphery of ablation zones, where exposure to temperatures that are not immediately lethal results in irrecoverable cellular injury [61]. These cells may subsequently die by apoptosis , or programmed cell death . In cells that avoided disruption of the plasma membranes and cellular active transport, intracellular damage to mitochondria can signal activation of caspases [62]. The expression of these proteins leads to apoptosis, which is characterized by cell shrinkage, membrane blebbing, chromatin condensation and genomic fragmentation [63, 64]. A handful of studies showed that cell death by necrosis is evident in the central part of the cryogenic lesion, while apoptosis is evident 8–16 h later at the periphery of the lesion [48, 62, 65, 66].
5.4.2 Indirect Cellular Injury
The second mechanism by which cryoablation kills cells is a result of cold-induced changes to the microenvironment, specifically the blood vessels. Intracellular ice formation within blood vessels causes damage to the vascular endothelial cells [67]. During thawing, the damage to the endothelial cell junctions causes increased capillary permeability, edema and congestion. Reperfusion brings in platelets, which contact the damaged endothelium and cause thrombosis and ischemia [68]. This loss of blood supply then results in additional necrosis.
5.4.3 Impact of Cell death on Immune Response
How the cells die is not only relevant to the clinical efficacy of cryoablation, but also the potential immune response . Apoptosis and necrosis are the primary mechanisms of tumor cell death and have significantly different effects on the immune response [69]. Necrosis , as caused by ice crystal formation and membrane damage , is characterized by release of intracellular contents. Many of these intracellular contents can be immunostimulatory, especially heat shock proteins (HSP) , DNA and RNA, which are recognized by Toll-like receptors , or “danger signals ” such as uric acid or the chromosomal protein HMGB1 (high mobility group box chromosomal protein 1), which can further activate the innate immune response [70]. The immune system may also be alerted to massive cell death not only by factors emanating from dying cells, but also from disruption of tissue architecture, such as fibrinogen, oligosaccharides of hyaluronan, extra domain A (EDA)-containing fibronectin and heparin sulfate proteoglycan [71–74]. Several studies have demonstrated that necrotic cells will lead to increased DC maturation and macrophage activation [75, 76].
Apoptotic cells do not release their contents as do necrotic cells, but rather are taken up by macrophages and dendritic cells without stimulating an immune response . In fact, several studies have shown that apoptosis does quite the opposite, leading to the suppression of an immune response [69, 77]. This makes some sense as apoptosis occurs physiologically in many tissues, and their uptake may be one mechanism by which the body maintains “self” versus “non-self.” The continual transport of apoptotic “self” cells and presentation of self-antigen may relate to peripheral tolerance [50, 78]. The recognition and phagocytosis of apoptotic cells is mediated by a large number of receptors and opsonins which bind to cellular ligands exposed on the surface of apoptotic cells. This not only prevents the release of the intracellular contents, but also modulates phagocyte function, inhibiting proinflammatory cytokine release and increasing TGF-B1 production [79, 80]. Dendritic cells that take up apoptotic cells have suppressed cytokine production and do not mature [81, 82]. These non-mature DC not only do not stimulate an immune response, but can trigger clonal deletion and anergy [77]. Defects in the manner by which apoptotic cells are cleared have been associated with the development of autoimmune diseases [69].
These observations are summarized by the Matzinger Danger Theory , as developed by Polly Matzinger and Ephraim Fuchs, which questions the self/non-self theory of immune response [83, 84]. The danger theory supposes that the generation of an immune response is not simply a matter of self and non-self, but also dangerous and not dangerous. The self/non-self theory argues that two signals are needed to generate an immune response; (1) recognition of the peptide antigen , presented on an antigen presenting cell, with the T-cell receptor and (2) the interaction of co-stimulatory molecules on the APC cell surface and T-cell. The danger theory argues a third “danger” signal is needed, and these are primarily provided by foreign substances (exogenous danger signals) or intracellular contents (endogenous danger signals) (Table 5.2). In the absence of danger signals, particularly with apoptosis, not only is an immune response not stimulated, but immunosuppressive cytokine release may direct the immune response in the opposite direction.
Table 5.2
Danger signals released after necrosis
Exogenous danger signals | Endogenous danger signals |
|---|---|
Lipopolysaccharide | Cytokines (TNFa, IL-6, IL-1b, IFNa) |
Lipoteichoic acid | ATP and UTP |
Lipoarabinomannan | Heat shock proteins |
Lipopeptides | Long unmethylated CpG sequences |
Peptidoglycan | Breakdown products of hyaluronan |
Mannans and mannoproteins | DNA and RNA |
Viral capsids | Uric acid |
Unmethylated CpG and dsRNA | HMGB1 |
This picture is not completely clear, however, and some studies have suggested that apoptotic tumor cells may be superior to necrotic cells in stimulating an anti-tumor immune response [85–87, 89]. This is likely secondary to superior phagocytosis by dendritic cells of tumor cell-derived apoptotic bodies, as compared with necrotic cells, and thus cross-presentation of antigens to CD8+ T-cells [85, 90]. Failure to clear apoptotic cells may lead to a secondary necrosis of uncleared cells, and pro-inflammatory signals [91]. It has been hypothesized that while the uptake of apoptotic cells is normally immunologically silent (or suppressive), the uptake of apoptotic cells by DC in the presence of inflammatory or danger signals from necrosis is the ideal situation for cross-presentation of antigen and priming of effector T-cells. Therefore, death primarily by necrosis may generate a humoral response, death primarily by apoptosis may generate immune tolerance, while death by a combination of necrosis and apoptosis, as is seen with cryoablation, may lead to a combined humoral and cellular response.
5.5 Technical Factors That Impact Cryoablation
As opposed to some of the other ablative technologies, there are multiple factors in cryoablation that can impact not only the successful ablation of the targeted therapy, but also the relative contributions of osmotic shifts, extracellular and intracellular ice formation, and vascular injury to cell death . Some of these factors are related to the tissue of interest and the anatomy, while others are operator-dependent. As these may alter the relative fractions of necrotic and apoptotic tissue, these factors may help to influence the resulting immune response .
5.5.1 Tissue Temperature and Duration of Freezing
The tissue temperature is a key factor in cryoablation . While several studies have shown that temperatures below - 20 °C efficiently kill cancer cells, other studies suggest temperatures below - 60 °C are needed [92]. The multiple methods by which cryoablation kills cells make determination of the exact lethal temperature for cells difficult to determine. Generally, investigators have set the lethal temperature in the - 40 to - 50 °C range. In this range, a high percentage of cells are likely to be destroyed by direct cell injury, with the indirect injury widening the kill zone over the warmer freezing temperatures. However, cancer cells seem more resistant to freezing than non-neoplastic cells, and so it is likely more important that multiple freeze-thaw cycles be employed than freezing to a specific temperature . The duration of freezing refers to the amount of time that the tissue is kept in the frozen state. This seems to be less of a factor when tissue is frozen to − 50 °C, but longer durations may improve cell killing in areas at the - 10 to - 25 °C range because of solute effects and recrystallization [93].
5.5.2 Rate of cooling and Thawing
The cooling rate refers to how quickly the temperature is dropped within the target tissue. This is dependent upon the distance from the cryoprobe. Tissue close to the heat exchange surface of the cryoprobe is frozen rapidly. As discussed, extracellular ice formation and osmotic changes occur more commonly with slower freezing rates, and so are less common near the probe. Intracellular ice crystals, however, form over a wide range of cooling rates, including slow rates, and thus will be a component of cell injury as the distance from the probe increases [94, 95]. The rate of cooling is in part determined by the tissue type and proximity to blood vessels, so that ultimately an equilibrium is met between heat loss and heat gained from blood supply. Experiments in vivo have shown that the cooling rate, whether slow or rapid, is less important than other factors in killing cells [75]. However, by altering the relative contributions of necrosis and apoptosis, it may be important in the type of immune response generated [46].
As discussed, the thawing of the tissue can be equally destructive as the freezing. Lessons learned from the clinical management of frostbite show that rapid warming increases the chance of cell survival. The longer the duration of the thaw, the greater the damage to the cells primarily due to an increase in the solute effects as well as increased size of the intracellular ice crystals [96, 97].
5.5.3 Multiple Freeze-Thaw Cycles
Several of the early studies of cryoablation have emphasized the need to repeat the freeze-thaw cycle [98–100]. Secondary to the damage to the cell membranes induced by the first cycle, the second cycle often leads to faster and more extensive tissue cooling. This serves to enlarge the volume of frozen tissue and pushes the border of certain disease destruction closer to the outer limit [92]. The second freeze-thaw cycle is best performed after a slow thaw, as this enlarges the size of the intracellular ice crystals. This assures a more lethal effect in the warmer freezing temperature zone at the periphery of the targeted tissue [101]. This may also serve to increase necrosis and decrease apoptosis within this peripheral zone, which may impact the immune response .
5.6 Studies Examining the Immune Response to Cryoablation
5.6.1 Studies Showing a Positive Benefit to Cryoablation
In some of the first publications to document the cryoimmunologic response, Ablin et al. [88, 102–104] and Shulman et al. [105–108] documented the humoral response triggered by cryoablation across a variety of models (rabbits, monkeys), documenting the presence of serum antibodies that recognized organ or tumor specific proteins after cryoablation . These investigators were among the first to suggest that cryoablation of tumors may be considered a form of immunotherapy and may be equally effective to tumor vaccines .
Subsequent pre-clinical studies of cryoimmunology explored the possibility that freezing a tumor and leaving it in place could render the animal resistant to a re-challenge. Studying both VX2 carcinoma in rabbits and sarcoma180 in ICR mice, Tanaka was able to demonstrate a tumor-specific resistance to re-challenge after cryoablation [25]. Neel et al. [29] also used two murine models to demonstrate this effect; the mammary tumor virus (MTV) in C3H/HeN mice, and 3-methylcholantrene induced sarcoma in CDF1 mice. Mice either underwent surgery or cryoablation . Tumor specific immunity, as measured by resistance to re-challenge, was consistently greater with cryosurgery than with surgical excision. Blackwood and Cooper also examined the response triggered by cryosurgery in both myosarcoma (MT449 A) and carcinosarcoma (Walker 256) in Wistar and Sprague-Dawley rats respectively [28] Again, cryosurgery , as compared to surgical excision, resulted in an immune response capable of preventing re-challenge and causing regression of second tumors. Bagley et al. [30] compared surgery to cryosurgery using MCA-10 fibrosarcoma in C57BL/6 mice, harvesting splenic lymphocytes at weekly intervals after treatment for cytotoxicity assays. They did demonstrate that mice undergoing cryoablation had significantly higher cytotoxicity than mice undergoing surgery or untreated mice. Cytotoxicity assays against other tumor types with different antigens showed no effect, demonstrating that the heightened immunity after cryosurgery was tumor specific. Sabel et al. [43] looked at MT-901 mammary adenocarcinoma tumors in BALB/c mice treated by cryoablation or surgical resection. After re-challenge, 86 % of mice treated by surgery developed second tumors compared with only 16 % of mice treated by cryosurgery. This was tumor-specific, as cryosurgery offered no protection against challenge with another cell line.
Evidence for a cryo-immune response is not limited to animal studies. In the 1970s and 1980s, based on the case reports of metastatic disease regressing after prostate cryoablation, investigators began to look for clinical evidence of an immune response. Several isolated studies at that time documented increases in relatively non-specific markers of immune response among patients undergoing cryoablation of oral cavity cancers [109–111] rectal cancers [112, 113] or breast cancer [24].
Although the clinical use of cryosurgery for cancer ablation has significantly expanded in recent years, there have been relatively few studies examining the immunologic impact in humans. In an interesting study of patients undergoing treatment for colorectal metastases to the liver, Ravindranath et al. [114] measured both the level of serum tumor gangliosides and their antibody titers after cryosurgery , radiofrequency ablation (RFA) or surgical excision. The level of serum gangliosides was significantly increased after cryosurgery but not after RFA or surgery. Likewise, only cryosurgery led to an increase in the IgM titer against tumor gangliosides. The authors concluded that cryosurgery-induced necrosis of the tumor not only released these gangliosides into circulation but also served as an adjuvant to the humoral response as repeated immunization with purified gangliosides failed to elicit an antibody response.
Si et al. [115, 116] studied 20 patients undergoing prostate cancer cryoablation and looked for immune responses against the human prostate cancer cell line LNCaP. They reported that 4 weeks after treatment there was both an increase in cytolytic activity against LNCaP, and an increase in the number of IFN-g producing T-cells, as measured by Elispot against tumor protein lysates. They also reported increases in TNF-a and IFN-g levels, with no changes in IL-4 or IL-10 levels.
More recently, Thakur et al. [117] reported a pilot study of cryoablation and GM-CSF for patients with renal cell carcinoma metastatic to the lung. Prior to cryoablation, an interstitial injection of GM-CSF (250 mg) near a selected lung metastasis was performed and then cryoablation was performed. Four days after cryoablation, aerosolized GM-CSF (250 mg/dose) was given for 1 week. After a three-week interval, a second treatment cycle was initiated. Although there were too few patients for statistical analysis, several measures suggested that the combination of cryoablation and GM-CSF induced an immune response, including an increase in PBMC cytotoxicity against RCC cell lines, an increase in the number of IFN-g producing T-cells by Elispot, and increased serum antibodies against RCC cell lines. In the case of this study, however, there were no controls that received GM-CSF alone, so it is not clear whether these effects were due to the cryoablation alone, the GM-CSF alone, or the combination.
5.6.2 Studies Showing No Immune Benefit, or Immune-Suppression
Several studies attempted to document an immune response to cryoablation but failed to do so [31, 35]. Müller et al. [35] using Dunn osteogenic sarcoma in C3H mice, showed that cryosurgery was superior to surgery in regards to metastases formation. Despite this finding, the authors could not document any differences in immune parameters between the cryosurgery and surgery groups, including NK function, T-cell cytotoxicity or antibody response. In a prostate cancer study in Copenhagen rats, Hoffman et al. [95] examined the effect of cryosurgery on secondary tumor growth after re-challenge, as well as attempted to document an anti-tumor antibody. Although there were anti-tumor antibodies detectable after cryosurgery , there was no significant impact on secondary tumor growth. Surmising that freezing normal prostate might generate immunity to prostatic antigens shared by both normal and malignant prostate tissue, Friedman et al. [118] found that freezing the normal ventral prostate of Copenhagen rats, combined with intralesional injection of Complete Freund’s Adjuvant (CFA) did not confer a protective immunity against a prostate cancer challenge using Dunning R3327 prostate adenocarcinoma. However, in Friedman’s study, normal prostate was ablated. Lubaroff et al. [119] performed a similar study, except in this case; Dunning R3327 tumors were created in the rats and frozen in combination with BCG. This did confer long-term immunity in 50 % of the rats.
More concerning have been some studies that didn’t just fail to document a benefit, but actually showed a negative effect [33, 34,37, 38, 120, 121]. The majority of these studies involved fibrosarcoma cell lines in rats. Hayakawa and colleagues, using a chemically induced fibrosarcoma, found mice treated by cryoablation had a decreased resistance to a secondary tumor challenge, as well as increased growth and metastatic rates of secondary tumors [34]. Shibata et al. [122], examining WKA fibrosarcoma in rats, found that pulmonary metastases established 1 day prior to treatment of a subcutaneous tumor, were enhanced by cryoablation . In contrast, using the same tumor type in a double grafted tumor system, cryosurgery did inhibit the development of contralateral tumors. This effect, however, did not appear to be T-cell dependent, as the anti-tumor activity of splenocytes was decreased in the cryosurgery group. In a follow-up study, the authors found that the anti-tumor resistance of rats was diminished by the adoptive transfusion of splenocytes from tumor-bearing mice treated by cryoablation, suggesting that the immunosuppression following cryosurgery might be caused by suppressor T-cells. Another animal study of the cryoablation of fibrosarcoma, this time in Sylvian golden hamsters, also suggested an increase in suppressor T-cells following cryosurgery [36].
5.7 Studies Examining Impact of Time and Technique on Immune Response
The conflicting reports regarding the immune response to cryoablation; some demonstrating a positive effect with others demonstrating a negative effect, raised concerns regarding the clinical use of cryoablation in cancer treatment. Adding to the controversy, several studies have shown that both stimulation and suppression may be the result of cryoablation, depending on the time-point you look for a response, and the technique used to perform the cryoablation .
Some studies have shown both stimulation and suppression at different time points after cryoablation . Misao et al. [32] compared cryosurgery and surgical resection in Sprague-Dawley rats implanted with a metastasizing comedo-type breast adenocarcinoma (MRMT-1). Mice were re-challenged after successful local therapy. When mice were examined 1–3 weeks after treatment, the surgical group demonstrated a superior rejection rate. However, by week 10, mice treated by cryosurgery demonstrated significantly better tumor rejection (80 %) compared to mice that had surgical excision (18 %). Lymph node metastases were also lower in the cryosurgery treated group. In a follow-up study, Maya et al. [123] examined the regional lymph nodes in these animals at varying time-points. Looking at paracortical hyperplasia and germinal center hyperplasia in the nodes as reflective of T-cell and B-cell activity, both increased by 1 week after treatment and remained high until 10 weeks. Macrophage activity, as measured by sinus histiocytosis, was increased by 3 weeks and also remained high. However, while PHA-induced proliferation of T-cells in the regional lymph nodes increased with cryoablation, it decreased in the peripheral blood at first, recovering to preoperative levels by 6 weeks. Atrophy of the thymus correlated with this as well. The authors concluded that there was an early tumor suppression that took place systemically as a result of cryosurgery, although this eventually reversed, leading to a high resistance to re-challenge with time.
It may be that not only is the time-point that you look for a response important, but also the method used to perform the initial cryoablation . Across the multiple studies, there were not only a variety of animal models being examined, but also variations in how cryoablation was performed, including the application of liquid cryogens directly to the tumor, contact cryoablation (where a cryoprobe is touched to the periphery of an exposed tumor), and percutaneous cryoablation (where the ice-ball is propagated from the center of the tumor outward). As these technical factors are associated with changes in minimum temperatures reached, cooling rates and thawing rates, and these impact the mechanisms of cell death, it is possible that the conflicting immunologic results may represent differences in technique.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree