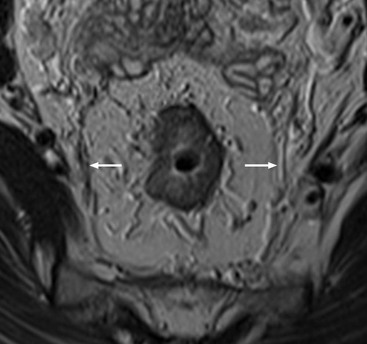
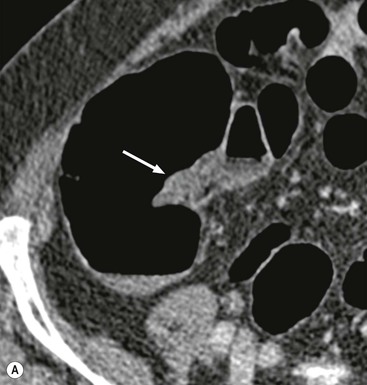
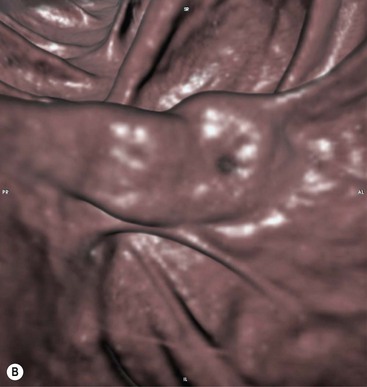
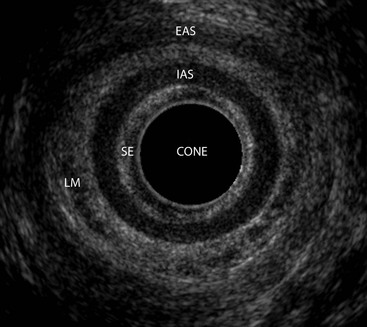
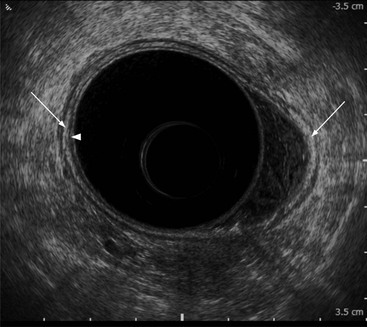
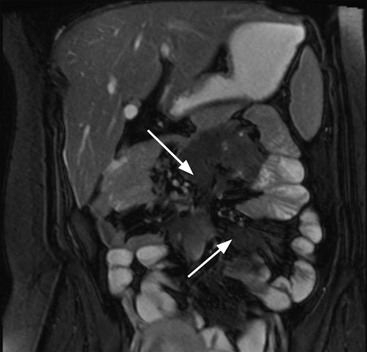
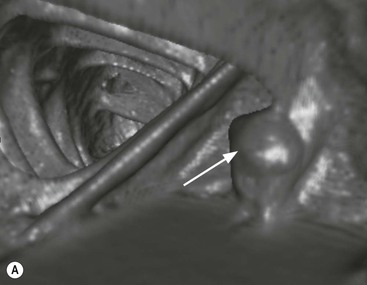
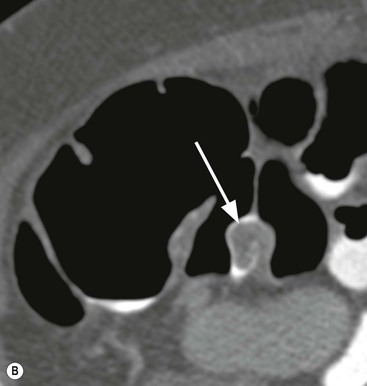
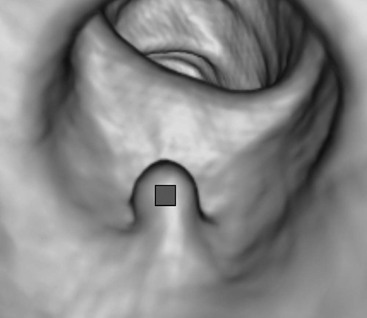
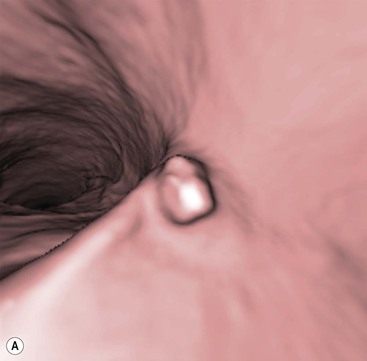
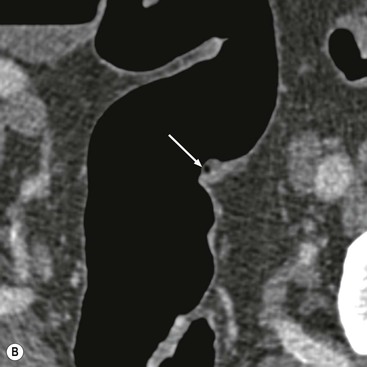
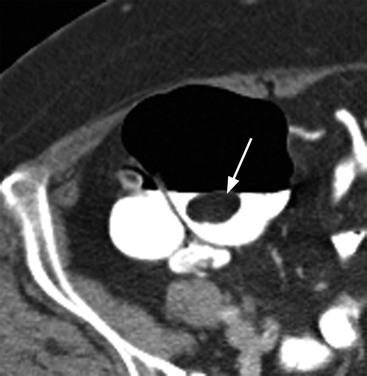
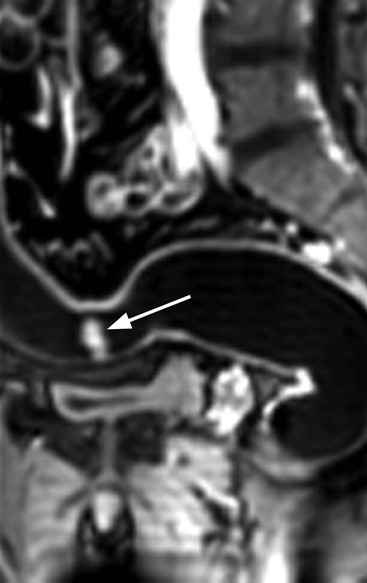
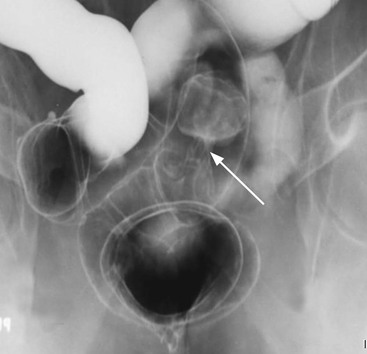
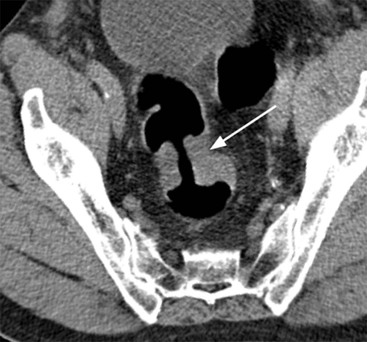
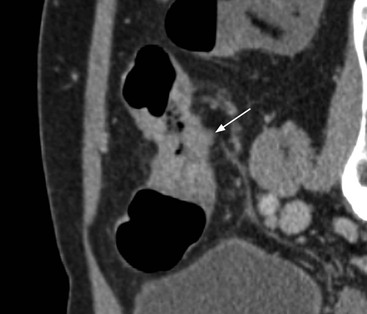
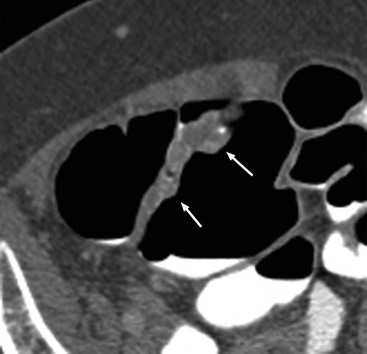
Stuart A. Taylor, Andrew Plumb The large bowel comprises the colon, rectum and anus. Knowledge of cross-sectional radiological anatomy of the colon is of increasing importance given the requirement for accurate pre-treatment staging of malignancy. The caecum, ascending colon and descending colon are covered anteriorly by visceral peritoneum and approximately 50% of the posterior aspect of these segments is retroperitoneal. The retroperitoneal colon has an adventitial layer, separating muscle from peritoneal fat. The anterior peritoneum runs medially onto the rudimentary mesocolon and laterally onto the abdominal wall as parietal peritoneum.1 The transverse and sigmoid colon have a mesentery formed from a double layer of visceral peritoneum sandwiching connective and adipose tissue with vessels, nerves and lymphatics. The outer muscularis propria of the colon has two layers, an inner circular and an outer longitudinal, with the myenteric (Auerbach’s) nerve plexus in between. The outer layer is thin, except where it is condensed into three narrow bands called the taeniae coli that contain more collagen and elastic tissue than muscle. Three rows of haustral sacculations arise between the taeniae, with haustral clefts between the sacculations. In the distal colon, haustra form only when the taeniae contract. The intraperitoneal colon is covered by mesenteric serosa. Subserosal fat in the caecum and sigmoid accumulates in small peritoneal pouches to form the epiploic appendages that may encase diverticula in the sigmoid. The superior mesenteric artery supplies the colon proximal to the splenic flexure via the ileocolic, right and mid-colic branches. The colon distally is supplied by the left colic, sigmoid and superior rectal artery branches of the inferior mesenteric artery. The marginal artery is a vascular arcade in the mesenteric border giving off short branches, the vasa recta, which penetrate the muscle layer close to the taenia mesocolica, and long branches entering between the taenia omentalis and libra. The veins and lymphatics follow the course of the arteries, draining up into the portal vein and celiac nodes, respectively. The mid-rectal veins drain into the internal iliac vein and so into the systemic circulation via the inferior vena cava. In the rectum, lymph drains superiorly via superior rectal artery nodes to the inferior mesenteric chain, posteriorly by nodes around the median sacral artery, and laterally around the middle rectal artery to the internal iliac chain. The rectum is defined as beginning at the third sacral level, although the sacral promontory is often taken surgically as the reference point. Others define it as the distal 15 cm of large bowel before the anus. Anteriorly the rectum is covered by peritoneum to the level of the junction of the upper two-thirds and lower one-third. The lateral and posterior aspects of the upper rectum and all the lower one-third are surrounded by the mesorectum, which is composed of loose adipose connective tissue containing the small perirectal lymph nodes and the superior rectal vessels. The mesorectum itself is enclosed by the mesorectal fascia (Fig. 29-1).2 Posteriorly the mesorectal fascia is separated from the presacral fascia by the thin retrorectal space; anteriorly it blends with the urogenital septum (Denonvillier’s fascia/rectovaginal septum), superiorly it is contiguous with the sigmoid mesentery, and inferiorly it terminates close to the anus in the parietal fascia covering the levator ani. There is no haustration in the rectum, but the valves of Houston create folds in the rectum. The presacral space is normally less than 1 cm at the fourth sacral segment, as measured from a lateral pelvic view during double-contrast examination, but may be up to 2 cm in the elderly and obese. The anus has a complex sphincter arrangement with an internal sphincter of smooth muscle (a continuation of the circular muscle coat of the distal rectum) and an external sphincter of striated muscle. Between these, the longitudinal layer, comprising striated and smooth muscle with extensive fibroelastic tissue, anchors the anus in position. The vascular subepithelial tissues seal the canal to maintain continence. The pelvic floor is an anatomical and functional unit and consists of muscles and connective tissue in three contiguous supporting layers. From cranial to caudal, these include the fascial layer or endopelvic fascia, the intermediate pelvic diaphragm and the urogenital diaphragm. The muscular pelvic diaphragm consists mainly of the levator ani complex. The colonic mucosa is columnar in type with goblet and some enterochromaffin cells arranged in crypts (of Lieberkühn). The surface pattern consists of fine parallel grooves running transversely with short intercommunicating branches, and is called the innominate groove pattern. The lamina propria contains lymphoid follicles, the submucosa, adipose tissue with neural elements (Meissner plexus), blood vessels and lymphatics. The mainstays of colonic radiological investigation are intraluminal contrast examinations and cross-sectional techniques. Although cross-sectional imaging is largely replacing contrast studies, the latter still have a role in specific clinical situations. A water-soluble contrast enema (Gastrografin diluted 3 : 1 with water or Urografin 150; Schering AG, Berlin, Germany) allows real-time evaluation of colonic anatomy and is most commonly used to test the integrity of surgical anastomoses, define colonic calibre, for example in suspected megacolon, delineate colonic fistulae and exclude mechanical obstruction. These agents are hypertonic and produce diarrhoea. Proximal to colonic strictures, water shifts will distend the colon and perforation resulting from this has been reported; care must be taken not to overfill the bowel proximal to a stricture. Isotonic contrast agents produce less fluid shifts and are increasingly used. A double-contrast barium enema (DCBE) involves full bowel preparation, an IV smooth muscle relaxant, partial filling of the colon with a barium suspension and insufflation of air or carbon dioxide to distend the colon. The objective is to acquire a series of images so that the entire colon is seen in double contrast, with no segment obscured by a barium pool or coated poorly. CT can be performed either with (CT colonography) or without gaseous distension of the colon – in general, gaseous distension improves diagnostic accuracy for colonic abnormality, although it is more invasive and not always appropriate in acute situations. Standard abdominal CT protocols may include colonic opacification using orally administered contrast (2% barium or Gastrografin suspension), typically commencing the day before the examination, together with IV contrast medium administration. Rectal contrast agents may be used, for example in suspected perforation or appendicitis. The colonic wall should be not more than 3 mm thick. The pericolic fat should be homogeneous with only a few vascular channels. The normal colonic mucosa enhances after intravenous contrast administration, but the pattern of mural enhancement in abnormal colon may provide clues as to the underlying diagnosis (Table 29-1). CT colonography (CTC) describes CT of the gas-distended colon. The use of oral contrast agents to tag residual colonic contents as higher attenuation is becoming mainstream, and can be combined with full or reduced laxative preparation, or without any additional laxative (‘prepless CTC’). High osmolar contrast agents such as Gastrografin have a laxative effect and can be used as a single colonic preparatory agent. Distension of the colon is preferably performed using carbon dioxide and automated insufflation devices give superior distension to manual techniques.3 Distension is improved with IV hyoscine butylbromide (Buscopan, Boehringer, Ingelheim, Germany).4 Supine and prone acquisitions (or decubitus views, if prone positioning is not possible) are essentially mandatory to ensure full mucosal visualisation. Review is performed using a combination of two-dimensional (2D) axial and multiplanar reconstructions, together with three-dimensional (3D) endoluminal reconstructions. Cross-correlation between 2D and 3D images is required to differentiate normal colonic structures such as the ileocaecal valve (Fig. 29-2), haustral folds and faecal residue from pathological entities. Collimation with multidetector CT is typically 1–2.5 mm. Intravenous contrast administration is not required for colonic evaluation. MR colonography (MRC) follows similar principles to CTC, and is most commonly performed after bowel purgation, although non-laxative approaches are possible. Colonic distension is achieved with 1.5–2 L warm water or gas (carbon dioxide or air). Bright lumen MRC uses a gadolinium-spiked water enema and typically a 3D T1-weighted spoiled GRE sequence is used. Dark lumen MRC uses air, carbon dioxide or water and is more widely performed, although it requires the administration of intravenous gadolinium.5 Evacuation proctography (EP) is a study of the dynamics of rectal evacuation. Conventionally the procedure has been performed using X-ray fluoroscopy, but MRI proctography is gaining increasing acceptance. The rectum is distended using thick barium paste (fluoroscopy) or air/ultrasound jelly (MRI). During the fluoroscopic procedure, a video recording is made of the voluntary evacuation of the paste. The small bowel should be opacified with a dilute barium suspension to show any enterocele, and some advocate contrast filling of the bladder and vagina. No such extracolonic organ opacification is required for MRI proctography and evacuation is captured using a rapid dynamic sequence such as true fast imaging with steady-state precession. Proctography may be viewed in three stages: rest, evacuation and recovery. At rest, the anorectal junction is normally just above the plane of the ischial tuberosities. Evacuation is initiated by 3 cm descent of the pelvic floor, widening of the anorectal angle, and relaxation of the anal sphincters. The rectum distal to the main fold is squeezed by raised intra-abdominal pressure against the levator ani to form a ‘zone of evacuation’ that empties in less than 30 s. On MRI proctography, organ prolapse is conventionally measured with respect to the pubococcygeal line.6 Abdominal ultrasonography requires a graded compression technique for good views of the colon. Gas distension often prevents visualisation of both walls, but the haustral pattern of the anterior wall should still be seen. Usually only the low reflective muscularis propria and reflective submucosa are identified. The bowel wall thickness may be measured, Doppler flow assessed and the pericolic tissues interrogated. Endosonography allows higher frequency probes to be used (10–20 MHz range) to show the wall layers in detail. The sonographic pattern is created by a mixture of interface reflections between, and reflections from, the thin layers. A four-layer pattern is seen in the anal canal (Fig. 29-3), with a five-layer pattern in the rectum (see Fig. 29-4). A polyp is an elevated mucosal lesion (Table 29-2). The majority occur sporadically in the general population, although there are many rare polyposis syndromes. The most clinically significant polyps are adenomas. By definition, adenomas contain dysplasia i.e. intra-epithelial neoplasia. Colorectal cancer (CRC) represents extension of this neoplasia beyond the muscularis mucosae into the submucosa. Perhaps two-thirds of CRC originates via an adenomatous precursor (the adenoma-carcinoma sequence).7 Detection of polyps is therefore clinically important, particularly as their removal substantially reduces subsequent carcinoma risk. TABLE 29-2 Classification of Polyps and Polyposis Syndrome Adenoma prevalence depends largely on age, gender and family history. They are rare under the age of 30, but are seen in around 30% of individuals over 50 years. They are commoner in men than women and a first-degree relative with colorectal cancer confers about 50% additional risk. Around 50% occur in the rectosigmoid, 25% in the descending colon, 10% in the transverse and 15% in the caecum and ascending. Right-sided adenomas increase in older subjects.8 Certain factors increase the chance of invasive malignancy in an adenoma. Size is most important; the risk of invasion in a series of over 11,000 adenomas was negligible for <5 mm polyps, 2% for polyps between 0.6 and 1.5 cm in diameter, 19% for 1.6–2.5 cm polyps, 43% for 2.6–3.5 cm polyps and 76% for polyps of >3.5 cm.9 Morphology is also important. Pedunculated polyps, which have a stalk separating the dysplastic epithelium from the deeper submucosa are often considered cured once resected, if the neoplasia is confined to the polyp’s stalk (Haggitt levels 1, 2 or 3). Sessile polyps have a similar diameter at their base and near the top—they are roughly hemispheric. These have an intermediate risk of invasive malignancy for a given size. So-called ‘flat polyps’ are not truly polypoid and hence are probably better termed ‘flat lesions’. A flat lesion is defined as being less than 2.5 mm in height above the colonic mucosal surface at endoscopy, or 3 mm on radiology.10 Flat lesions may be depressed rather than elevated relative to the colonic mucosa. Such flat depressed lesions carry a higher risk of invasive cancer for a given size, whereas other subtypes of flat lesions are generally less aggressive. They can be very challenging to detect, both at endoscopy and on imaging. Pathological subtype is a third key factor. Villous adenomas are higher risk than tubular or tubulovillous lesions. Barium enema may suggest a villous histology when the surface is lacelike or granular due to the trapping of barium in the villous interstices. Similar trapping of oral contrast may occur on CT, although imaging features in general are non-specific. Non-adenomatous polyps were once felt of lesser clinical importance due to a lower risk of malignancy, although this perspective is evolving. Traditionally, polyps were divided into adenomas, hyperplastic polyps and others (including juvenile, inflammatory, lymphoid and other rare polyps). Some polyps which were historically classified as hyperplastic have microscopic architectural distortion and are now termed sessile serrated adenomas. Their genes can show errors in sequences of repeated DNA, termed microsatellite instability (MSI) and/or gene hypermethylation (CpG island methylator phenotype, CIMP). They feed into a second major pathway of colorectal carcinogenesis termed the ‘serrated pathway’ which is hypothesised to account for up to 35% of CRC.11 This implies polyps other than conventional adenomas may have clinical significance. Furthermore, even true hyperplastic polyps may harbour some genetic alterations also seen in CRC and share many risk factors with adenomas and CRC. They may represent a marker that an individual is at higher risk of subsequent CRC or genuinely represent precursor lesions. Familial adenomatous polyposis (FAP) is caused by a mutation of the APC tumour suppressor gene on chromosome 5q21, and accounts for about 1% of CRC. Inheritance is autosomal dominant. APC is generally the first gene mutated in the sporadic adenoma-carcinoma sequence: individuals with FAP are therefore already one step along the pathway of tumourigenesis. More than 100 adenomas have to be present for the diagnosis; typically several hundred polyps are present. These may cause rectal bleeding, diarrhoea and mucus discharge. All affected patients eventually develop CRC. Preventative proctocolectomy is therefore recommended. Extracolonic polyps also occur. Gastric adenomas and hamartomas are present and almost 100% of FAP patients have duodenal adenomas clustered around the ampulla (possibly due to the co-carcinogenic effect of bile). There is a 5% risk of periampullary duodenal carcinoma, a major cause of death in those who have had proctocolectomy. Extraintestinal manifestations include multiple osteomas of the skull and mandible, epidermal cysts (often facial), congenital hypertrophy of retinal pigment epithelium, abnormal dentition and desmoid tumours. Gardner’s syndrome is a variant of FAP with prominent skeletal and skin manifestations. Desmoid formation is often precipitated by trauma or surgery. These benign fibromatous tumours are locally invasive and involve the abdominal wall, small-bowel mesentery or retroperitoneum. Previously, desmoid disease was a major cause of morbidity and mortality, although this has improved with advances in surgical expertise. In the early stages CT and MRI show ill-defined mesenteric infiltration with small-bowel tethering (Fig. 29-5), before mass development (which may be huge). Imaging often underestimates the extent of disease at surgical resection. Hereditary non-polyposis colorectal cancer (HNPCC) is an autosomal dominant condition caused by faults in DNA mismatch repair (MMR) genes and probably accounts for 5% of all CRC. The lifetime risk of CRC in HNPCC is 70–85%. Genetically, these tumours exhibit high levels of microsatellite instability (MSI). Other tumours are also increased, notably of the endometrium, small bowel and renal pelvis/ureter. Clinical criteria for this condition follow the ‘3-2-1 rule’: (A) three or more relatives with a HNPCC-associated cancer, (B) two or more successive generations affected, (C) one or more tumours diagnosed before the age of 50 years, (D) one should be a first-degree relative of the other two. Cancers occur at an earlier age in HNPCC (mean 45 years); about 70% are in the proximal colon and multiple tumours are common. Small polyps at an early age may be present. This autosomal dominant condition is characterised by mucocutaneous pigmentation and intestinal hamartomas, mainly in the stomach and small bowel. Large-bowel polyps are fewer, but are larger, often pedunculated, and may bleed. There is an increased risk of gastrointestinal tract cancer, including CRC. Extraintestinal cancers are also increased, particularly of the ovary, cervix, thyroid, testis, pancreas and breast. There are a number of other rare polyposes. Turcot’s syndrome is an association between colorectal adenomas/carcinomas and primary brain tumours. It can arise via a HNPCC-type defect in mismatch-repair genes or FAP-type defect in the APC gene. Sebaceous gland tumours occurring with HNPCC-type tumours is called Muir–Torre syndrome. Cowden’s syndrome is an autosomal dominant trait with hamartomatous intestinal polyposis and lesions of the skin, mucous membranes, breast, thyroid and dysplastic cerebellar gangliocytoma. Juvenile polyposis is also autosomal dominant and presents in infancy with multiple juvenile hamartomatous polyps in the stomach, small bowel and colon. CRC risk is increased. Cronkhite–Canada syndrome is a diffuse intestinal polyposis that is associated with alopecia, hyperpigmentation of the skin and nail atrophy secondary to gross malabsorption. Serrated polyposis (previously known as hyperplastic or metaplastic polyposis) is a very rare condition with increased CRC risk and is diagnosed when there are multiple or large, proximally located, serrated colonic polyps. CTC is the most accurate radiological technique for polyp detection, surpassing double-contrast barium enema (DCBE)12 and approaching that of colonoscopy for larger polyps.13 The entire colonic surface must be visualised using either a primary 2D approach with 3D problem-solving or a primary 3D endoluminal fly-through with 2D problem-solving (Fig. 29-6). The exact analysis method used is less important than the training and experience of the reporting individual.14 Computer-aided detection (CAD) increases sensitivity with only a small reduction in specificity and should be used as a second reader to maximise its benefit (Fig. 29-7).15 Once a polyp candidate is detected, it must be interrogated further to confirm its nature. Faecal residue may mimic a polyp; variable attenuation due to internal gas content is a distinguishing feature from the usual homogeneous soft-tissue attenuation of a polyp (Fig. 29-8). Faecal tagging (use of oral contrast to label or ‘tag’ residual colonic contents) improves both sensitivity and specificity; lesions which might be obscured by retained liquid residue can be seen within the higher-density fluid (Fig. 29-9) and tagged stool will not be mistaken for a polyp due to its high attenuation. Stool often moves between the prone and supine acquisitions. The mesenteric colon may be mobile and changes in colonic position between the supine and prone acquisition may suggest ‘movement’ of a polyp; use of fixed landmarks such as diverticula or haustral folds may be helpful. Lipomas may be polypoid on 3D but the fat density on 2D is diagnostic (Fig. 29-10). Diverticula are seen as extraluminal pockets of gas. An inverted diverticulum may simulate a polyp on 3D, but on 2D the gas content indicates its true nature. Flat lesions can be very hard to detect and may only manifest as subtle wall thickening on 2D images (often best appreciated on an abdominal window setting) or minor protuberance or irregularity on 3D images. CAD can help detect flat lesions16 and newer CAD algorithms will improve performance further. Dark-lumen sequences depict polyps as enhancing protrusions from the normal mucosa (Fig. 29-11). Endoluminal projections are feasible but may appear pixelated due to the current lower spatial resolution of MR. As CTC rises in popularity, so DCBE declines. However, substantial numbers are still performed and interpretation skills will be needed for the foreseeable future. Polyps en face create a ring shadow with a sharp inner border and a fading outer margin. Viewed obliquely, a protruding polyp creates the ‘bowler hat sign’, which points towards the colonic lumen. The stalk and target signs are features of pedunculation. The stalk is outlined by two parallel lines of barium that run obliquely to the axis of the lumen, distinguishing it from a haustral fold (Fig. 29-12). The ‘target sign’ is created when the head and stalk are superimposed. A polyp may create a filling defect in the barium pool, assuming it is not too deep. Primary CRC is the third commonest cancer in the UK with around 40,000 new cases a year (2008 figures). It is the second commonest cause of cancer death. The lifetime risk is 1 in 15 for men and 1 in 19 for women. The risk increases with age, with almost three-quarters of cases seen in people aged 65 or more. Family history is a factor in around 20% of cases of CRC. Other risk factors include obesity, cigarette smoking and chronic inflammatory bowel disease. Anti-inflammatory medications such as aspirin and NSAIDs may be protective, as may diets low in red meat and high in fibre. Just over half of all CRCs arise in the rectum or sigmoid; the rectum alone accounts for one-third of cases. Overall 5-year survival is about 50%. Major prognostic factors include local tumour stage, vascular or lymphatic invasion, preoperative elevation of carcinoembryonic antigen (CEA) and tumour differentiation (grade). Colon cancer and rectal cancer are currently treated slightly differently. The mainstay of therapy for both is surgical excision, but in the rectum it is more difficult to achieve adequate clearance margins to prevent local recurrence whilst avoiding significant complications. The static nature and pelvic position of the rectum, however, make it amenable to chemoradiotherapy, which has been shown to decrease local recurrence in later-stage disease. Local staging is therefore particularly important in rectal cancer. Both colon and rectal cancers can be staged according to the Dukes’ and TNM systems (Table 29-3). CTC has equivalent sensitivity to colonoscopy for detecting colorectal cancer,17 although one advantage of colonoscopy is the ability to obtain biopsy confirmation of malignancy. CTC depicts annular or semi-annular lesions with shouldered ends and luminal narrowing (Fig. 29-13). Flat or plaque-like cancers show wall thickening and irregularity (Fig. 29-14). At conventional CT, malignancy is usually seen as an area of focal wall thickening (>3 mm), often with extension into the pericolic fat and local nodal enlargement (Fig. 29-15). Tumours are usually homogeneous but may be heterogeneous in the context of large adenocarcinomas or mucinous tumours or when associated with abscess formation. Mucinous tumours, both primary and metastatic, also have a propensity to calcify. CT can estimate the T stage, with accuracy for identification of disease extension beyond the bowel wall of around 86%.18 Colonic segments with a mesentery are fully enveloped by visceral peritoneum and hence tumours here are more likely to achieve T4a status (Fig. 29-16
The Large Bowel
Anatomy
Radiological Investigation
Tumours
Polyps
Histological Type
Single or Few in Number
Polyposis
Epithelial
Adenoma—tubular, villous, tubulovillous
Familial adenomatous polyposis, Turcot’s syndrome
Adenocarcinoma
Cowden’s disease
Hamartomatous
Juvenile
Juvenile polyposis
Metaplastic
Peutz–Jeghers syndrome Metaplastic polyposis
Inflammatory
Post-inflammatory polyp
Post-inflammatory polyposis
Nonepithelial
Lipoma, carcinoid, GIST, benign lymphoid, neurofibroma
Lymphomatous polyposis, Metastatic, neurofibromatosis
Miscellaneous
Endometriosis
Cronkhite–Canada syndrome
GIST = gastrointestinal stromal tumour.
Polyposis Syndromes
Familial Adenomatous Polyposis.
Hereditary Non-polyposis Colorectal Cancer (Lynch Syndrome).
Peutz–Jeghers Syndrome.
Rare Polyposes.
Radiographic Features of Polyps
CT Colonography.
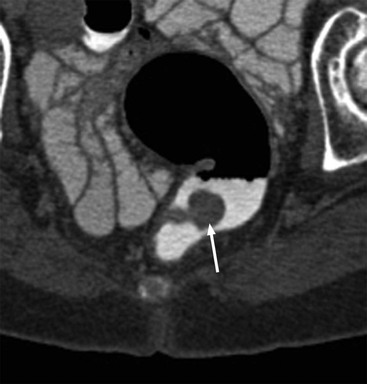
MR Colonography.
Double-Contrast Barium Enema.
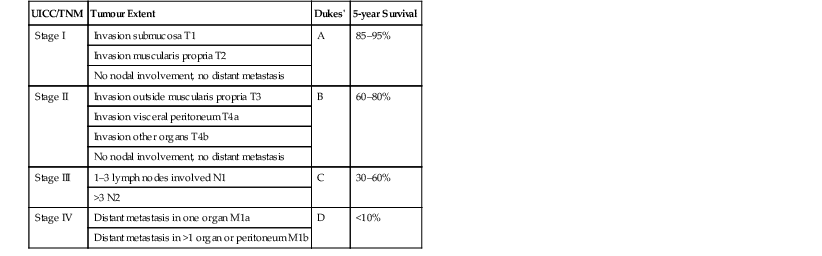
Colorectal Cancer
Colon Cancer
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Large Bowel
Chapter 29