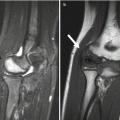
Fig. 12.1
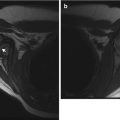
Intertrochanteric fracture in a 15-year-old girl whose leg tangled in a swing as she fell. The fracture (arrow) required plate and screw fixation
Femoral head dislocations are largely posterior (Fig. 12.2). Most hip dislocations in children can be treated with closed reduction. However, post-reduction radiographs should be evaluated for joint space asymmetry, as soft-tissue interposition may prevent congruous hip reduction and necessitate open reduction. The labrum, capsule, lateral acetabular apophysis, avulsed ligamentum teres, and osteochondral fragments may become entrapped [4]. If there is joint space asymmetry after closed reduction of hip dislocation, computed tomography (CT) can help evaluate for intraarticular osteochondral fragments and subtle acetabular fractures, whereas MRI best evaluates soft-tissue interposition. Although MRI cannot predict avascular necrosis, it can help characterize this after hip fracture or dislocation [5].
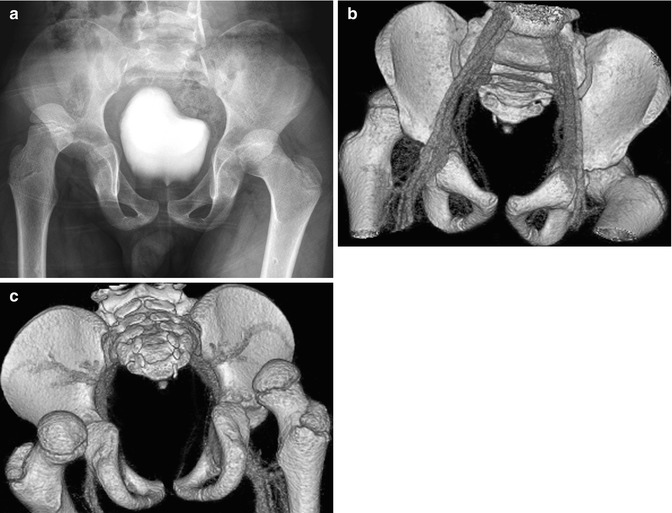

Fig. 12.2
Bilateral acute posterior hip dislocations in a 6-year-old boy after a motor vehicle accident. (a) Both hips are dislocated, and there is no obvious fracture. Anterior (b) and posterior (c) views from contrast-enhanced three-dimensional (3D) computed tomography (CT) reformation with surface rendering more clearly display the relationship of the dislocated femoral heads to their respective acetabula
2 Apophyseal Injuries
Overuse injuries of the hip are increasing as more adolescents become involved in year-round competitive sports. Apophyseal injury around the hip is most commonly encountered in the mid- to late teen period, after the long bone growth spurt. At this time, the physis is the most biomechanically vulnerable component of the muscle-tendon-bone axis (whereas in skeletally mature individuals, similar stress typically leads to injury of the tendon or the myotendinous junction instead). The ischial tuberosity at the hamstring tendon attachment site is most often affected [6] (Fig. 12.3). Other apophyses that may be injured include the anterior inferior iliac spine (insertion site for the rectus femoris); the anterior superior iliac spine (sartorius); the iliac crest (abdominal oblique); and the lesser trochanter (iliopsoas) (Figs. 12.4, 12.5, and 12.6). Chronic repetitive stress may lead to apophyseal strain, presenting as dull, activity-related pain near the hip. An acute apophyseal avulsion fracture, on the other hand, presents as acute, localized pain and swelling, exacerbated by palpation or stretching.
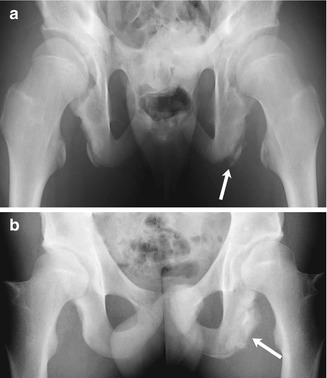
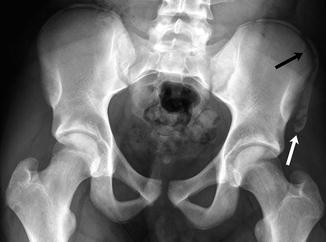
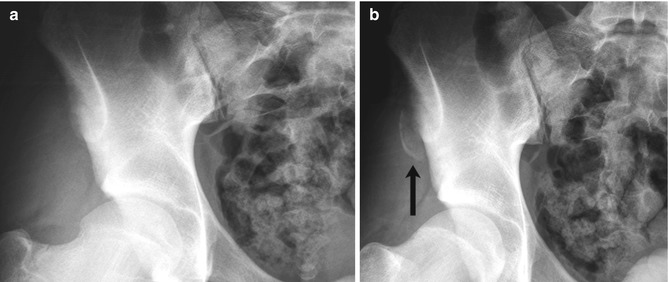
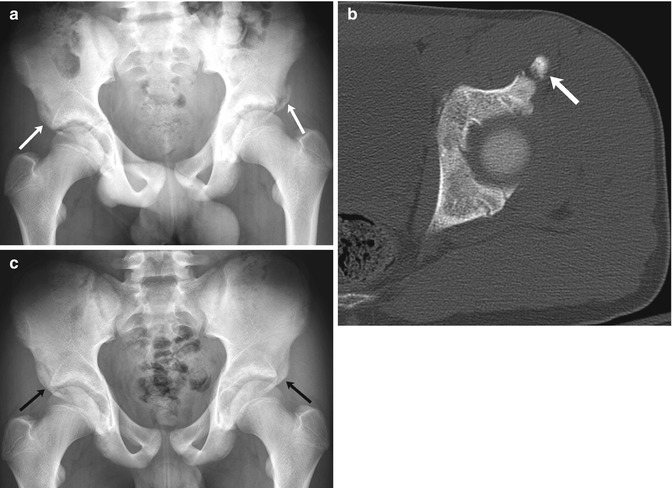

Fig. 12.3
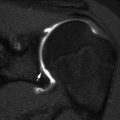
Avulsion of the ischial tuberosity in a 14-year-old soccer player with left hamstring pain for 3 months. (a) Outlet view shows avulsion of the left ischial tuberosity (arrow) with irregularity and indistinctness of the underlying bone margin. Ossification center on the right is normal. (b) Three years later, frontal radiograph shows mature exuberant bone production at the avulsion site (arrow), whereas the ossification center on the right has fused

Fig. 12.4
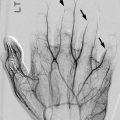
Acute avulsion of the anterior superior iliac spine in a 15-years-old who developed sudden, excruciating left hip pain while playing football. There is acute avulsion of the left anterior superior iliac spine (white arrow) as well as the lateral aspect of the iliac crest apophysis (black arrow)

Fig. 12.5
Avulsion of the anterior superior iliac crest in a 16-year-old boy who developed severe right hip pain and heard a “pop” while sprinting. The avulsion is hidden on the straight frontal neutral view (a) but is clearly seen (arrow) with frog lateral positioning and slight tilt of the pelvis (b)

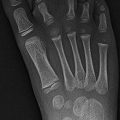
Fig. 12.6
Bilateral fractures of the anterior inferior iliac spine (AIIS) in a 13-year-old boy with sudden hip pain while playing soccer. (a) There is an acute avulsion fracture of the left AIIS and a healed avulsion fracture of the right AIIS as well (arrows). (b) CT at the time of injury shows mild displacement of the apophysis (arrow). (c) Four months later, bilateral AIIS fractures have healed and appear symmetric (arrows)
Chronic repetitive stress at the apophyseal growth plates about the hip may cause microfractures and secondary inflammation, akin to a Salter-Harris I stress fracture. However, an acute apophyseal avulsion fracture is caused by sudden eccentric muscle contraction, often during deceleration or landing. This leads to fracture through the growth plate and separation of the apophysis. These injuries are most commonly seen in adolescent athletes whose sports involve kicking, jumping, and rapid acceleration/deceleration such as soccer, football, gymnastics, sprinting, and ice hockey.
Apophyseal injuries are treated conservatively with rest and ice followed by gradual return to weight bearing and physical therapy. Surgical intervention is rarely needed but may be undertaken if a large, displaced fragment causes ongoing pain or if there is unstable nonunion.
Imaging
In chronic apophyseal strain, the physis may appear normal or mildly wide and irregular, but the apophysis is not displaced. If the ischial tuberosity has not yet fully ossified at the time of stress, a fracture fragment may not be evident but the bone margin may appear sclerotic and irregular, suggesting infection or tumor. With acute apophyseal avulsion, the apophysis is variably displaced. If the acute event is missed and imaging performed later, then well-rounded soft-tissue ossification may be seen near the expected location of the apophysis.
Ultrasound (US) can demonstrate tendonopathy associated with apophyseal strain, with a thickened and echogenic tendon insertion. CT is rarely needed for diagnosis or treatment planning, but it can clearly show the pelvic avulsion fracture. Especially in cases that are unclear clinically, MRI may be useful to demonstrate edema in the apophysis and adjacent bone and sometimes in the enthesis as well (Fig. 12.7).
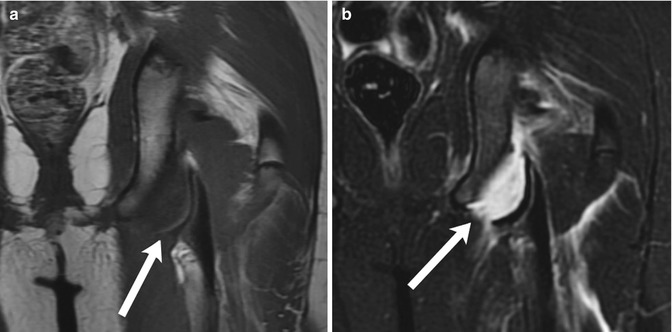

Fig. 12.7
Avulsion fracture of the left ischial tuberosity. (a) Coronal T1-weighted (T1-W) magnetic resonance image (MRI) demonstrates separation of the cortex (arrow) at the left ischial tuberosity from the underlying bone. (b) Coronal short tau inversion recovery (STIR) image shows fluid in the gap between the two bone fragments (arrow), along with edema in the surrounding musculature (Courtesy of J. Herman Kan)
Although overuse injuries are more common in adults, they are also seen in athletic adolescents. These include femoral neck or pelvic stress fractures (Fig. 12.8), tendonosis involving the iliopsoas and adductor tendons, the iliotibial band “snapping hip” syndrome, athletic pubalgia (Fig. 12.9), and labral tears.
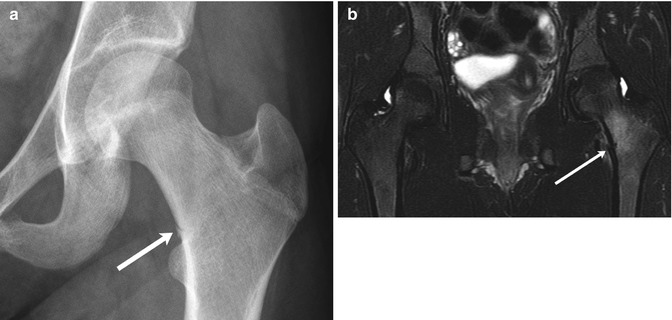
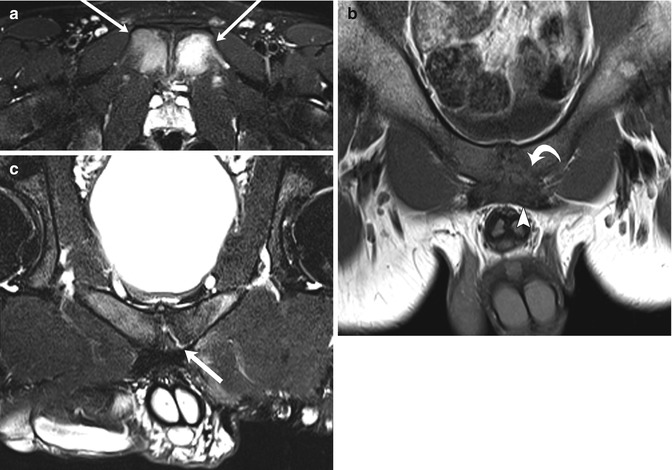

Fig. 12.8
Stress fracture in a 15-year-old girl with history of an eating disorder, who presented with left hip pain after increasing the intensity of her cross-country training. (a) There is a stress fracture (arrow) in the medial cortex of the base of the femoral neck. (b) Coronal T2-weighted (T2-W) fat-suppressed (FS) magnetic resonance (MR) image shows mild cortical offset at the fracture site (arrow) with surrounding bone marrow and soft-tissue edema

Fig. 12.9
Athletic pubalgia in two teenage male soccer players with groin pain. Axial T2-W FS (a) and coronal oblique T1-W (b) images of a 14-year-old boy show severe osteitis pubis (arrows), with midline pubic plate injury involving detachment of the left side of the rectus aponeurosis (arrowhead) and pubic apophysis (curved arrow). Coronal oblique T2-W FS image (c) in a 19-year-old man shows a left-sided sub-apophyseal aponeurosis injury, with fluid cleft (arrow). Athletic pubalgia is seen in young adult high-level athletes (Courtesy of Adam Zoga)
3 Slipped Capital Femoral Epiphysis
3.1 Epidemiology
Slipped capital femoral epiphysis (SCFE) typically develops in obese young adolescents, and, although it can result from local infection or trauma, it usually occurs in response to routine activity. This shearing disruption of the growth plate and subsequent slip of the femoral head is most often encountered during periods of rapid growth. The epiphyseal slip thus affects boys about 2 years later (age 12–15, median 13 years) than girls, who generally exhibit symptoms before menarche. SCFE is the most common hip abnormality of adolescence, with an annual incidence of approximately 11 per 100,000 [7]. The condition occurs almost twice as often in boys as in girls, possibly due to their more vigorous activity and a longer and more intense growth spurt. Incidence increases in warm weather, suggesting that environmental trauma may be a factor. One-half of patients have a history of significant injury antecedent to diagnosis of the epiphyseal slip. At presentation, approximately 10 % of slips are bilateral.
Several other demographic features are of interest. For unknown reasons, SCFE is nearly four times more common in black children than in white children in the United States [7]. The condition is also more frequently seen in Native Americans, Hispanics, and Asian/Pacific Islanders compared to white children. Regardless of race, incidence is much higher in those who are overweight, with more than 80 % of children diagnosed with SCFE having a body mass index greater than the 95th percentile [8]. The incidence of SCFE has increased over the past 40 years, mirroring the trend of childhood obesity [9]. In addition, skeletal maturity is often mildly delayed. Although 40 % have a bone age within one standard deviation above the mean, few are more mature than this and 60 % are below the mean. The combination of being overweight and skeletally immature means children with SCFE are often both short and heavy. SCFE in a tall, thin child is quite unusual.
3.2 Etiology
Although the predisposing factors are known, the actual precipitating event and pathogenesis of epiphyseal slip are not well understood. Because SCFE is often linked to the adolescent growth spurt, a hormonal etiology or substrate is likely, but investigation has found that growth hormone levels in these patients are normal. Nonetheless, there is a substantial increased risk for SCFE in children with endocrinopathies, especially those with hypothyroidism and those treated with growth hormone. SCFE occasionally appears to be familial [10].
During adolescence, the shear strength of the upper femoral growth plate increases and becomes more dependent on the internal anatomy of the growth plate than on the perichondral complex. In overweight children, forces generated by usual activity (which can be up to six to ten times body mass) are sufficient to produce shear fracture of the proximal femoral growth plate experimentally. After epiphyseal slip, the otherwise normal growth plate shows a fracture-like cleft meandering through the degenerative zone and the cartilage-bone junction [11].
The biomechanical stresses that most likely produce growth plate shear are abduction and external rotation, especially in extension; decreased femoral anteversion may also play a role. The muscles around the hip insert into the region below the greater trochanter laterally and in a spiral fashion. All but the adductors pull the shaft anterolaterally and encourage external rotation. If a shear fracture occurs through the growth plate, the femoral epiphysis will remain within the acetabulum, appearing to be displaced posterior and medial with respect to the upper femur. However, it is actually the femoral shaft that has moved anterolaterally.
Idiopathic SCFE must be distinguished from the pathologic growth plate and metaphyseal fractures that occur in renal osteodystrophy with secondary hyperparathyroidism and that follow radiation therapy. Careful evaluation will show the actual slip in these cases to be within the metaphysis, not at the growth plate.
3.3 Presentation
Diagnosis of idiopathic SCFE can be difficult, as early symptoms are often vague, including mild weakness, fatigue, or limp. About one-half of children with SCFE present with hip pain, while one-fourth complain of knee pain. Onset of symptoms can be abrupt or insidious, developing over several months, and it is common for diagnosis to be delayed by several months. In cases that progress slowly, the child may walk with a limp, hold the leg in external rotation, and have limited, painful internal rotation. If onset of symptoms is sudden (in the setting of acute athletic trauma), pain is usually severe, and there is often marked epiphyseal displacement.
3.4 Prognosis
With subacute to chronic symptoms, diagnosis may be delayed, especially if the child is initially evaluated in the primary care setting. This is unfortunate, since delayed diagnosis may result in increased slippage (Fig. 12.10). Once identified, the child with SCFE needs to be closely evaluated for bilateral disease. Both hips will eventually be involved in 20–80 % of cases [8]. Most contralateral slips develop within 1 year of diagnosis. Contralateral slip is most common in younger children and has been found in all children whose SCFE occurred before age 10. Girls less than 12 years old and boys less than 14 are also at increased risk [12]. The modified Oxford bone age score, based on features of the proximal femur and acetabulum, can more reliably predict risk of subsequent contralateral slip, with less skeletally mature children at greater risk (Table 12.1) [13, 14]. For example, the risk of a subsequent contralateral slip in a child with a modified Oxford score of 16 is about 85 %. Postoperative weight loss in obese children can reduce the risk of contralateral slip [15].
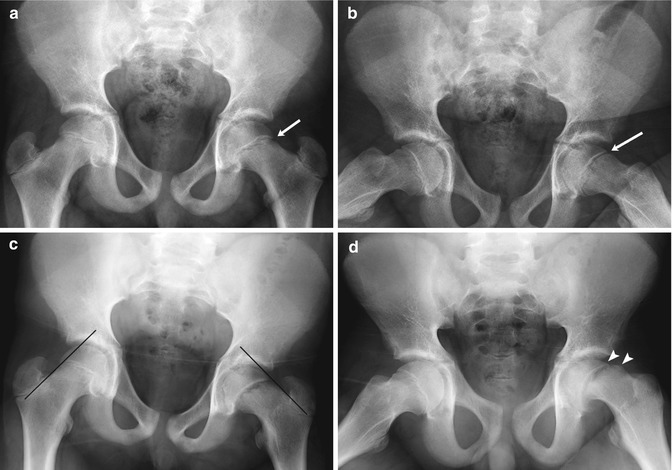

Fig. 12.10
Slipped capital femoral epiphysis (SCFE) in a 14-year-old boy with intermittent hip pain for several months, presenting with increased left hip pain after falling off his bicycle. (a) (neutral), (b) (frog lateral). Subtle left hip physeal widening without femoral head offset (arrows) was initially missed. (c) (neutral), (d) (frog lateral). Eleven days later, after another bicycle fall and increased left hip pain, the slip is much more evident. No portion of the left femoral head lies lateral to a line drawn along the femoral neck tangent, but a small amount of the normal right femoral head does. There is now approximately 25 % offset of the left femoral head and neck (arrowheads), still mild according to the Wilson grading method
Table 12.1
Modified Oxford method of assessing skeletal maturity
Location | Point score | Determining features |
|---|---|---|
Femoral head | 5 | Proximal femoral neck straight |
Fovea poorly defined | ||
Beak at distal medial epiphysis | ||
6 | Epiphysis wider than neck | |
Fovea clearly defined | ||
7 | Physis closing | |
Greater trochanter | 4 | Peak or nodule tops greater trochanter |
5 | Space between nodule and femoral neck smoothly filled with bone | |
6 | Physis closing | |
Lesser trochanter | 3 | Physis open |
4 | Physis closing | |
5 | Physis closed | |
Triradiate cartilage | 1 | Open |
2 | Partially closed | |
3 | Closed | |
Ilium | 3 | No ossification of iliac crest apophysis |
4 | Apophysis beginning to ossify |
Depending on their ability to bear weight and/or the results of orthopedic manipulation on evaluation, hips are considered either “stable” (able to walk with/out crutches) or “unstable” (unable to walk). Treatment, as described below, consists primarily of in situ stabilization with a screw across the physis. For unstable hips presenting less than 24 h after onset of symptoms, gentle repositioning can be attempted prior to fixation. Early complications include slip progression, hardware failure, avascular necrosis, and chondrolysis. Unstable SCFE has a much higher incidence of avascular necrosis (up to 50 % in some series) compared with stable SCFE (nearly 0 %), likely due to retinacular vascular injury at the time of initial displacement [16]. Other risk factors for the development of avascular necrosis include (a) over-reduction of an acute (unstable) SCFE or attempting reduction of a chronic SCFE, (b) placement of pins in the superolateral femoral head, or (c) performing a femoral neck osteotomy for correction of SCFE [17]. Avascular necrosis is radiographically evident within 1 year after the slip [17].
Chondrolysis, a rapid loss of cartilage in the hip joint, follows SCFE in a small minority of patients (see section below). Although not etiologically related, chondrolysis can coincide with avascular collapse of the femoral head and usually occurs within 1 year of the epiphyseal slip. Chondrolysis is common after severe epiphyseal slips but sometimes occurs in the immobilized normal hip during treatment of the opposite side. It occurs in untreated as well as surgically operated hips, especially if fixation hardware penetrates the cartilage. The etiology of chondrolysis with SCFE is unknown. Synovial and capsular fibrosis and adhesions could potentially lead to a decrease or alteration in the synovial fluid with subsequent impairment of cartilage nutrition. Immunologic changes have been documented but may be secondary rather than causative.
Late complications of SCFE include osteoarthritis, “pistol-grip” deformity leading to femoroactabular impingement (see section below) (Fig. 12.11), and limb length discrepancy due to premature closure of the growth plate. Epiphyseal slip during adolescence is thought to predispose adults to osteoarthritis. For chronic slips in general, approximately three-fourths of patients will have good outcomes, whereas one-fourth will progress to premature osteoarthritis. However, the long-term prognosis for pinned mild chronic slip is excellent.
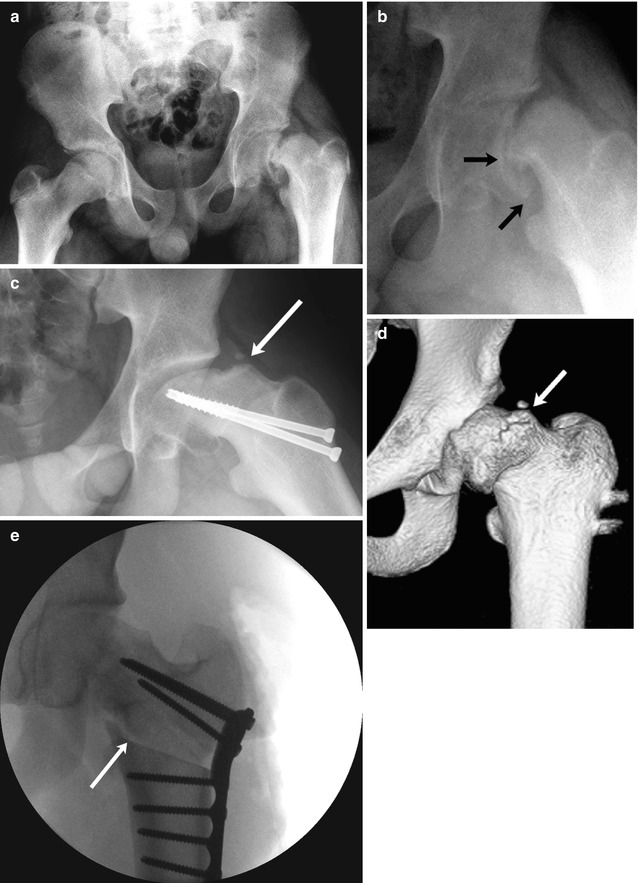

Fig. 12.11
SCFE and resultant femoroacetabular impingement in a 15-year-old boy complaining of left hip and knee pain 3 months after falling off a porch. (a) (neutral), (b) (frog lateral). There is approximately 50 % slip of the femoral head in relation to the neck (small arrows), moderate to severe according to the Wilson grading method. The patient underwent immediate screw fixation (c, frog lateral). At 1.5 years follow-up, there is significant pistol-grip deformity of the femoral head and neck with a large bony bump along the lateral femoral neck (arrow), resulting in limited range of motion. (d) Preoperative 3D-CT also shows the bony bump (arrow) causing femoroacetabular impingement. (e) After proximal femoral osteotomy (arrow), alignment is improved
3.5 Treatment
Treatment of SCFE attempts to stabilize slip and prevent further displacement, stimulate early growth plate closure, and prevent complications such as avascular necrosis, chondrolysis, and osteoarthritis [18]. Manipulation and attempted reduction are associated with an increased incidence of avascular necrosis, although this approach may be considered in acute, unstable SCFE presenting less than 24 h after onset of symptoms. At least in theory, relieving acute kinking of epiphyseal blood vessels may restore blood flow.
Further slip is generally prevented by mechanical fixation of the femoral epiphysis relative to the femoral neck (see Fig. 12.11). A single cannulated screw is employed most often, though occasionally a second pin is placed if the slip is very unstable. Care should be taken to ensure that all stabilizing devices are in fact within the bone, not protruding through the femoral head into the joint space. Even with biplane right angle radiographs, this can be difficult due to the spherical nature of the femoral head. Sometimes CT is helpful, but artifact from hardware may limit its utility.
For severe SCFE with a high likelihood of developing femoroacetabular impingement, slip reduction using the modified Dunn procedure can be considered [19]. In this newer surgical technique, the hip is dislocated via greater trochanter osteotomy, preserving the blood supply to the femoral head to reduce the risk for avascular necrosis. Femoral neck osteotomies may also be performed at the time of original surgery or afterwards to realign the hip, but these are associated with increased incidence of avascular necrosis. Prophylactic contralateral pinning is commonly employed, especially if patients have known endocrinopathy, are young, or are likely to be lost to follow-up.
3.6 Imaging (Box 12.1)
Box 12.1: Imaging Features of Slipped Capital Femoral Epiphysis
Frontal radiographs | Wide physis |
No femoral head lateral to Klein line | |
Smaller femoral head due to rotation | |
Lateral radiographs (frog or true lateral) | Offset of femoral head relative to neck |
MRI | Wide physis |
Increased signal on fluid-sensitive sequences | |
Periphyseal edema | |
Joint effusion | |
Nuclear scintigraphy | Increased proximal femoral physeal activity |
Radiography
It is difficult to confirm mild epiphyseal slips solely with a frontal projection. Demineralization of the femoral head and neck is usually evident. The growth plate may appear wide, probably owing in part to angulation following the slip (see Fig. 12.10). Increased opacity, generally below the growth plate, results from density caused by either the slipped and superimposed base of the epiphysis or by reactive sclerosis in the metaphysis. While a line drawn along the lateral femoral neck (Klein line) in a normal patient will intercept the epiphyseal ossification center so that a small portion of femoral head lies lateral to this line, with SCFE there is either no femoral head ossification center lateral to the line or the amount is less than on the normal side (see Fig. 12.10). In addition, rotation may make the height of the epiphysis appear smaller than on the unaffected side. These signs are often quite helpful but may be equivocal, and a frog or true lateral projection may be necessary to confirm the diagnosis.
If an acute slip is suspected, a true lateral projection of the hip and pelvis avoids the stress of positioning the patient for the frog lateral position, which may increase slippage. Unfortunately the resultant radiograph is often suboptimal in obese children. A modified rolled lateral hip view may be useful, as it is less painful than performing the frog lateral. In any of these lateral projections, the anterior and posterior corners of the epiphysis and metaphysis should closely match. Consistent displacement of these points indicates an epiphyseal slip. Occasionally, a second image with a rotated thigh is needed to confirm osseous offset.
If the epiphyseal slip is not recent, reactive bone will have formed along the medial and posterior femoral neck, buttressing the posteriorly and medially displaced femoral epiphysis. With very severe slips, the lateral portion of the femoral neck articulates with the acetabulum, and remodeling generally incorporates this into the functional femoral head. Premature fusion of the growth plate may result in femoral shortening.
Frog lateral radiographs can be employed to grade the severity of chronic slips [18] (Box 12.2). The Wilson method calculates the degree of femoral head displacement relative to the metaphysis (see Fig. 12.11). Less than one-third displacement is considered mild, one-third to one-half displacement moderate, and greater than one-half displacement severe. The Southwick method compares the angle between the femoral head and shaft on both hips in the frog lateral view. Subtraction of the head-shaft angle of the uninvolved hip from the head-shaft angle of the involved hip calculates the slip angle. Less than a 30° difference is mild, 30–60° moderate, and greater than 60° severe.
Box 12.2: Grading of Slipped Capital Femoral Epiphysis Using Frog or True Lateral Hip Radiographs
Grade | Wilson method: amount of femoral head displacement | Southwick method: slip angle |
|---|---|---|
Mild | Less than 1/3 | 0–30° |
Moderate | 1/3 to 1/2 | 30–60° |
Severe | Greater than 1/2 | Greater than 60° |
Nuclear Scintigraphy
In cases of hip pain with negative or equivocal radiographs, a technetium-99m methylene diphosphonate (Tc-99m MDP) bone scan can demonstrate early SCFE and increased proximal femoral physeal activity (Fig. 12.12). Pre- and postsurgical Tc-99m MDP bone scans can occasionally help predict development of avascular necrosis (AVN), as patients with decreased femoral head uptake are more likely to progress to AVN [20].
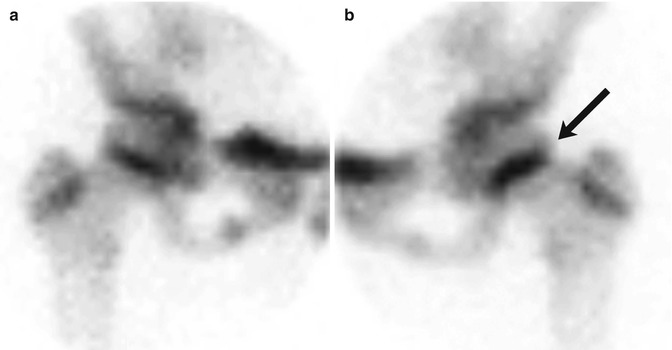

Fig. 12.12
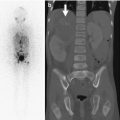
SCFE in an 11-year-old girl with left hip pain. Pinhole images of the normal right (a) and symptomatic left (b) hips from Tc-99 MDP bone scan show asymmetrically increased activity in the left proximal femoral physis (arrow) compared to the right. Subsequent evaluation confirmed the diagnosis of SCFE on the left
CT
CT is generally not employed for diagnosis, although it can be used to measure hip angles, to assess hardware position, and to aid in preoperative planning before capital realignment or femoral neck osteotomy [21].
MRI
MRI can be useful in cases with negative or equivocal radiographs [22, 23]. In such cases, MRI may demonstrate distortion of the physis as well as periphyseal bone marrow edema (Fig. 12.13). In both acute and chronic cases, coronal or axial T1-weighted (T1-W) images best demonstrate focal or diffuse widening of the physis along with globular or diffuse intermediate T1 signal in the growth plate. Hyperintensity of the physis on short tau inversion recovery (STIR) imaging is inconsistently seen. Joint effusion and synovitis are common. MRI can also help detect occult contralateral SCFE and assess later complications, especially labral injury related to femoroacetabular impingement (discussed below). Chondrolysis and AVN can also be characterized on MRI, despite susceptibility artifact from fixation hardware [24].
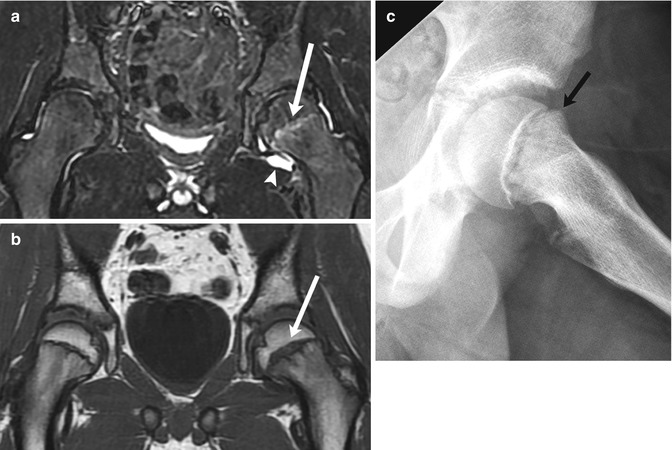

Fig. 12.13
Minimally displaced SCFE in an 11-year-old with left hip pain. (a) Coronal STIR image shows increased signal in the left proximal femoral physis (arrow) and a joint effusion (arrowhead). (b) Coronal T1-W MR image more clearly depicts physeal widening (arrow). (c) Subsequent radiograph confirms subtle femoral head and neck offset (arrow)
3.7 Differential Diagnosis
Early changes on radiographs may be subtle, so high suspicion for SCFE is necessary in any adolescent or preadolescent with hip or knee pain; a frog lateral or true lateral view of the hip is indispensible. The differential diagnosis for this pathology includes synovitis, arthritis, infection, early-stage AVN, myotendinous injury, and labral tear. However, imaging features are characteristic once the slip has progressed.
4 Avascular Necrosis
Ischemic osteonecrosis is usually referred to as avascular necrosis (AVN). However, circulatory disturbance is not necessarily complete and may only cause isolated osteocyte death, possibly preserving some marrow viability. Although it follows a similar reparative course in all epiphyseal areas, local factors and treatment will affect the rate and radiographic manifestations of healing. The basic etiology of ischemia may be unknown, as in Legg-Perthes (LP) disease, or well understood, as in the hemoglobinopathies and Gaucher disease. Either the major vascular supply or small intraepiphyseal vessels may be affected.
The pattern and development of the arterial supply to the femoral head helps elucidate the etiology of idiopathic AVN. Vessels originate from the medial and lateral femoral circumflex arteries, which form an anastomotic ring at the base of the capsule [25]. Those that penetrate the capsular attachment run sub-synovially along the neck of the femur to form an intracapsular network; several well-defined ascending cervical vessels supply the metaphysis and then pass peripherally to the growth plate and then the femoral head chondroepiphysis. The most important blood supply to the epiphysis comes from a single lateral ascending cervical branch (also called the lateral epiphyseal artery). This artery passes through a narrow interval between the greater trochanter and femoral neck, a prime location for potential compression and occlusion. A variable and small portion of the femoral head blood supply comes from the foveolar (ligamentum teres) artery.
Up to the age of 15–18 months, abundant arteriosinusoidal vessels pass through—rather than around—the growth plate. After this time, the bony plate of the ossification center creates a barrier, and vessels no longer penetrate the physis [26]. The vascular supply changes in other ways as well during early development. By age 2–3 years, the growth plate has become intracapsular, the lateral circumflex artery has assumed the role of supplying the greater trochanteric region, and the femoral head relies on the medial circumflex artery alone. Thus, the infantile pattern of multiple small vessels originating from both the lateral and medial circumflex arteries changes to a childhood pattern, in which a few large ascending cervical arteries (the posterosuperior and posteroinferior epiphyseal vessels) arise without significant anastomotic connections. In infancy, AVN generally requires occlusion of one large extracapsular vessel. This might result from compression in the treatment of infantile hip dislocation. Later in childhood, occlusion of a smaller—but relatively more important intracapsular branch—can produce AVN.
The actual occlusive event and ensuing bone death may be clinically unapparent. However, histologic changes are seen almost immediately as osteocytes disappear, leaving empty lacunae. Note that the process of bone infarction is radiographically occult and that radiographic changes actually reflect healing (with the exception of ossification center fractures). Epiphyseal resorption is not a sign of destruction but rather a sign of repair.
Imaging (Box 12.3)
Dead bone has no distinctive radiographic appearance and is indistinguishable in form and density from live bone [27]. Therefore, any change in appearance means that the process of healing has begun. As new bone lamellae are laid down over the dead trabeculae, a slight but definite increase in the opacity of the epiphysis develops; this is the first radiographic change in the femoral head (Fig. 12.14).
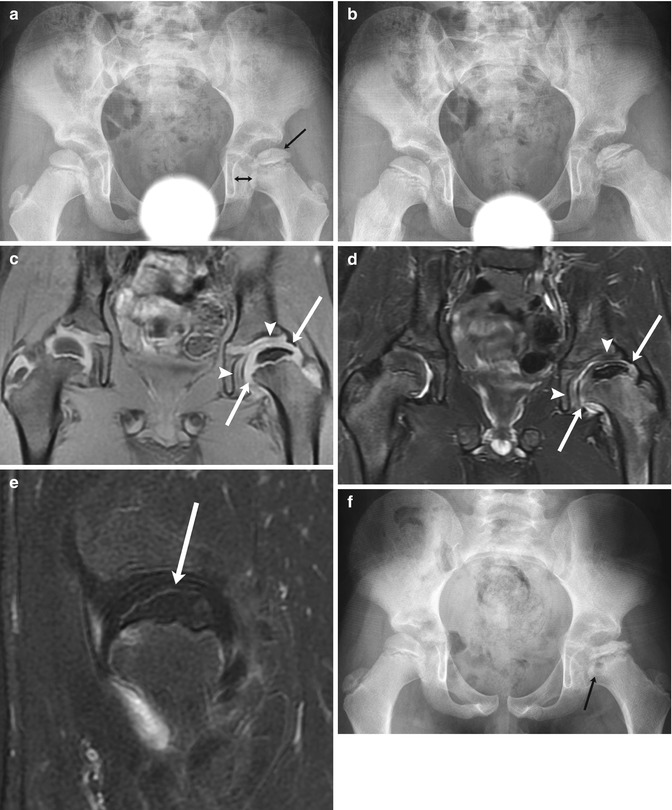

Fig. 12.14
Progressive findings of LP disease in a 7-year-old boy who presented with hip pain. At presentation (a (neutral), b (frog lateral)), there is mild flattening of the relatively dense left femoral epiphysis (arrow, a) and mild widening of the medial joint space (double arrow, a). Subtle fissuring is evident on the frog lateral view (b). Coronal T1-W (c) and T2-W (d) images 6 weeks later show no significant joint effusion, but acetabular (arrowheads) and femoral head (arrows) cartilage overgrowth accounts for joint space widening, and the epiphysis is small and diffusely hypointense. (e) Sagittal T1-W FS gadolinium-enhanced subtraction MRI shows the femoral head (arrow) does not enhance. (f) Four months later, at stage of intermediate healing, there is progressive flattening and early fragmentation of the femoral head. Multiple fracture lines are evident. Note metaphyseal lucency (arrow)
Box 12.3: Radiographic Changes of Avascular Necrosis of the Femoral Head
Phase | Pathology | Radiology |
|---|---|---|
Initial | Vascular occlusion and osteocyte death | Normal |
Early | Necrotic bone with beginning revascularization | Increased bone density |
Ossific growth impairment | Small ossific epiphysis | |
Cartilaginous epiphysis continues to grow | Increased medial joint space | |
Subchondral fracture of necrotic bone | Subchondral fissure | |
Early intermediate | Dead bone resorption | Intermixed lucencies (sequestra and immature bone) |
Fibroblast ingrowth | ||
Early new bone deposition | Resorption dominates | |
Late intermediate | Resorption slows | Gradual reconstitution of outline and integrity of ossification center |
Deposition predominant | Bone of mixed maturity | |
Late | Formation of mature Haversian systems | Remodeling of amorphous bone into mature trabeculae |
Lack of vascular supply to the ossification center prevents its continued normal growth. Growth of the cartilaginous epiphysis, however, is unimpaired, since it is nourished by synovial fluid. In fact, cartilage growth is stimulated by the insult at the ossification center, and hyperplasia of both epiphyseal and acetabular cartilage takes place. MRI or arthrography of both hips may demonstrate this cartilaginous overgrowth (Fig. 12.15). Increased cartilage along the medial aspect of the femoral head displaces the small ossification center laterally, increasing the distance to the bony acetabulum (the medial joint-cartilage space). Thus, although AVN is itself radiographically occult, secondary signs may be evident.
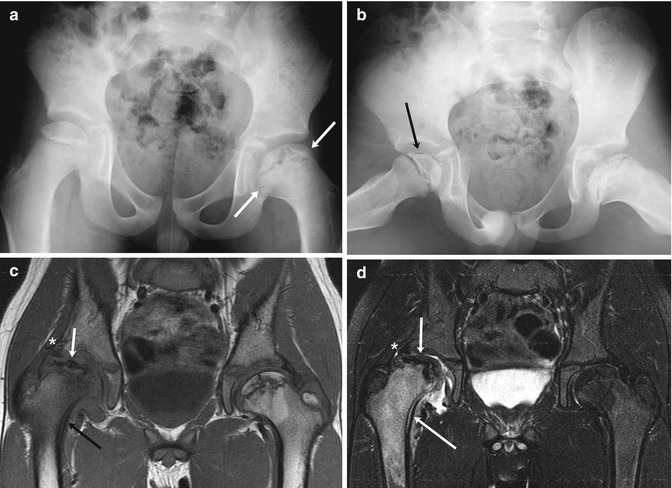

Fig. 12.15
Old left-sided and acute right-sided LP disease in a 6-year-old boy with 1 week of limp and right knee pain. (a) At presentation, the left femoral head is broad (arrows) and the femoral neck is short and wide, indicating previous LP disease. (b) The crescent sign of acute right-sided LP disease is seen only on the frog lateral (arrow), since it is in the anterior portion of the femoral head. Ten months later, coronal T1-W (c) and T2-W FS (d) MR images show marked fragmentation of the right femoral head (short arrows) with edema and periosteal thickening of the femoral neck (black arrow (c), long arrow (d)), indicating intermediate stage of healing. Also note deformed labrum (asterisk) as the femoral head is extruded. In contrast, the left hip is in the late stage, with normal fatty marrow signal in the remodeled femoral head
A fissure fracture is another common relatively early finding in AVN. The fracture occurs in necrotic bone rather than at the interface between dead and live bone. In the hip, the fissure is located anterolaterally in the epiphysis and is thus best shown on the frog lateral projection (see Fig. 12.15) (note that orthopedic surgeons usually refer to this as the “crescent” rather than “fissure”). It usually appears as a single thin, linear lucency extending over the lateral one-third of the epiphysis; it may extend in an arcuate fashion over most of the periphery of the ossification center. The fissure may vary in width, or it may spread, forming several fissures. It is often more lucent than a simple linear fracture, probably because the fracture line contains gas induced by the vacuum phenomenon. If this is the case, the fissure may seem to lie between the ossification center and the overlying cartilage. This type of fissure fracture line is a moderately early sign of AVN and becomes apparent only when other signs of osteonecrosis are present. Resorption often starts peripheral to this fissure, and, as the adjacent bone is removed, the line quickly disappears.
The vascular and fibroblastic ingrowth that supplied the osteoblasts for the new bone lamellae is also the source for the osteoclasts that begin the resorption of the necrotic bone marrow and trabeculae. The resorptive process starts anterolaterally and is seen as a gradual disappearance of bony trabeculae as they are replaced with fibrous material. Since the process is temporally and spatially both irregular and unpredictable, islands of bone may persist adjacent to large areas of fibrosis, leading to a granular, fragmented radiographic appearance. Large sections of normal or dense bone surrounded by lysis represent sequestra; these may persist unchanged for months or years.
Bone deposition follows resorption, but the rate is unpredictable. In some cases, resorption and re-ossification are so closely allied that they cannot be differentiated, whereas in others resorption is so complete that the ossific epiphysis vanishes before new bone formation becomes evident. The immature bone appears clumped and amorphous and is eventually replaced with mature bone trabeculae. The process from bone death to complete remodeling can be quite rapid in the very young (less than age 4 years) but typically lasts 2 or more years. It is not uncommon to find a saucerlike osseous deficiency at the vertex of the femoral head more than 5 years after the initial necrosis.
4.1 Legg-Perthes Disease
Legg-Perthes (LP) disease is an idiopathic osteonecrosis of the proximal femoral epiphysis. Sufficient evidence from both pathologic and nuclear imaging studies indicates that this is an AVN, but until the precipitating cause is recognized LP disease will remain a puzzling condition, distinct from well-understood avascular necroses. However, the arterial caliber in these children is unusually small; they also demonstrate an impaired hyperemic response when exposed to an ischemic stimulus [28]. LP disease may result from a hypercoagulable state.
Legg and Waldenström reported the disease in 1909, and in 1910 the descriptions of Legg, Calvé, and Perthes were published [29–32]. LP disease has been labeled “coxa plana” in an attempt to avoid eponyms, but this term is inaccurate, as progression to a flat femoral head is often avoidable with current treatment. “Femoral head osteochondrosis” or “idiopathic osteonecrosis of the femoral head epiphysis” are acceptable synonyms but are infrequently used. Legg-Perthes disease seems to be the most common eponym, but other permutations are employed.
Epidemiology
The epidemiology of LP disease is characteristic and suggests strong environmental factors in addition to genetic predisposition. Its incidence ranges from 5 to 15 cases per 100,000. Although bilateral in 13 % of cases, the likelihood of involvement of the second hip decreases if disease onset is after age 6 or 7. The disorder is four times more common in boys than in girls. LP disease is much more common in Caucasian children than in other ethnic groups. It is uncommon enough in children of African descent that investigation for underlying disorders is warranted. Independent of race, the disease is more prevalent in northern latitudes, with each 10° increase in latitude associated with an incidence increase of 1.44 [33]. LP disease is also associated with Down syndrome and minor birth defects. There is a strong correlation between LP disease and lower socioeconomic status and markers of deprivation such as low birth weight, short stature, and small foot size [34]. Maternal smoking and exposure to secondhand smoke have also been implicated.
The age range of LP disease is 3–12 years, with few patients seen at either age extreme and most first seeking treatment around age 5–8. Girls tend to present younger than boys. Markedly delayed skeletal maturation is common in both sexes and may be seen in siblings. It is very rare to have a bone age equal to or greater than the chronologic age, and almost three-fourths of boys studied have bone ages more than two standard deviations below the mean [35]. Maturation can plateau for as many as 3 years in some patients [36].
Presentation
While the appearance is distinctive, signs and symptoms of LP disease are not different from most afflictions of the hip. Groin pain, limp, spasm, decreased internal rotation, and thigh and buttock atrophy are characteristic. An early history of transient hip pain (but not toxic synovitis) may be found in less than 5 % of cases [37, 38].
In most, one or more of the initial episodes of infarction are neither clinically nor radiographically detectable. Instead, imaging findings are based on the reestablishment of the vascular supply, resorption of necrotic bone trabeculae and marrow, and subsequent re-ossification. Revascularization can occur within weeks by recanalization of the occluded vessel. However, the process of neovascularization sometimes takes months to years. The complete healing process typically occurs over a period of 2–4 years.
Prognosis
Prognosis depends on age at presentation. Children who are diagnosed before the age of 5–7 years have a significantly better outcome than those who present after eight or nine, likely because they have a longer time before maturity in which to heal and remodel. In general, the prognosis for girls is worse than for boys. Poor outcome is associated with being overweight and having progressive loss of hip movement, adduction contracture, longer duration from onset to completion of the healing phase, and worse clinical symptoms. Partial epiphyseal necrosis heals with less femoral head deformity and incongruity that do cases with total femoral head involvement.
Multiple classification systems for disease severity have been proposed over the years. The Catterall classification [39] divides the process into four categories:
(i)
Anterior involvement only, without collapse or sequestrum formation
(ii)
Greater involvement, with some collapse or sequestrum formation but with viable medial and lateral bony segments supporting normal epiphyseal height
(iii)
Large central sequestrum with collapse, but viable posterior segment and small medial and lateral supports
(iv)
Total or nearly complete collapse and resorption of the bony epiphysis
The problem with this classification is that 16–40 months must pass before a case can be assigned to a category. Indeed, if classification is attempted too early, 40 % of patients change category. In addition, considerable interobserver error is encountered with this system [40].
The lateral pillar classification system [41] is more reproducible (more than 80 % interobserver agreement) and has more prognostic value [42]. Similar to the Catterall system, this imaging scheme is applied only after the disease has progressed to fragmentation. The revised system uses the following groups, based on the loss of height observed in the lateral quarter of the femoral head or the lateral pillar:
Group A. Lateral pillar is normal.
Group B. Loss of lateral pillar height up to 50 %.
Group B/C border. Loss of lateral pillar height of 50 %.
Group C. Loss of lateral pillar height greater than 50 %.
A study evaluating outcomes for these different groups found that the prognosis for all lateral pillar group A hips and two-thirds of group B hips is excellent. However, the prognosis for group C and the group B/C border hips is generally poor. Only one in four group B/C border and only one in eight group B hips had good result [43].
The ultimate shape of the femoral head after reconstitution of the bony nucleus depends not only on the initial amount of necrosis but also on the intensity and direction of forces acting on the epiphysis during healing, when it is biologically plastic. The final result ranges from a round femoral head indistinguishable from the normal side to a grossly flattened, enlarged, and deformed articular segment (coxa plana and magna). The extent of deformity results from unusual growth patterns caused by muscular and weight-bearing forces acting on a weakened structure. The lateral portion of a subluxated femoral head is subject to increased pressure, resulting in deformity of both the osseous and cartilaginous components of the epiphyseal head. Lateral growth of epiphyseal cartilage also causes an increase in the growth plate’s circumference, widening the femoral neck in continuity with the enlarged head. In addition, periosteal new bone laid down along the femoral neck widens the proximal femur. Lateral extrusion of femoral head cartilage is easily detected on arthrography or MRI but may not be recognized on radiographs until epiphyseal ossification begins to appear superolateral to the outer edge of the femoral neck.
Injury to the vascular supply of the dividing cartilage cells at the growth plate may slow or completely halt growth, leading to premature fusion and resultant shortening of the femoral neck. The greater trochanter, with its separate blood supply, continues to grow by enchondral and appositional processes, shortening the normal articular-trochanteric distance (see Fig. 12.18 below). Thus, what appears as overgrowth of the trochanter is actually caused by lack of growth of the femoral neck. This configuration contributes to a Trendelenburg gait. Greater trochanter epiphysiodesis in younger children or distal transfer of the greater trochanter in skeletally mature children addresses this problem.
Separation of an osteochondral fragment at the vertex can occur as late as 18 years after treatment. This is an uncommon problem, encountered in only 2–4 % of patients. The mechanism is uncertain—perhaps a segment of bone never united with the main center or a late shear fracture occurred through a weak and partially ossified zone at the vertex. Separation is more likely with late-onset disease. One-third of the reported cases have been bilateral, suggesting that these cases may actually represent femoral head dysplasia of the osteochondritis dissecans type. There is both histologic and scintigraphic evidence that second episodes of infarction occur, but—unlike with AVN due to Gaucher disease—clinically apparent recurrence is uncommon.
Several different classification systems describe the radiographic appearance of late LP disease, all with the goal of predicting the likelihood of premature osteoarthritis. These include epiphyseal-femoral neck-acetabular measurements as well as various quotients, relationships, and tests for symmetry and roundness [44–47]. Catterall’s grading is usually adequate [39]:
Good: head essentially round, slight decrease in height acceptable
Fair: femoral head broadened, up to one-fifth uncovered
Poor: more than one-fifth uncovered, increased medial joint space, short neck
Complete coverage of the femoral head is not necessary for an acceptable outcome, since 80 % of normal children have some uncovering of the femoral head.
Overall, two thirds of patients are symptom-free about 30–40 years later, despite the presence of residual radiographic abnormalities in almost all patients treated with older modes of therapy. Those with residual effects may have mild limp, minimal leg shortening, and little or no pain or functional impairment. However, premature osteoarthritis may develop in some patients who have significant abnormal findings, such as aspherical femoral head with incongruent acetabulum, osteochondral fragment, labral tear, or trochanteric overgrowth. This is in marked contrast to patients with a history of developmental hip dysplasia, who may have severe symptoms despite relatively mild radiographic abnormalities.
Etiology
It is uncertain what causes interruption of blood supply to the femoral epiphysis in LP disease, but genetic and environmental factors probably contribute to the process. Thrombophilias such as protein-C, protein-S, or antithrombin III deficiencies, or Factor V Leiden mutations, have been inconsistently linked to LP disease. Abnormalities in the insulin-like growth factor I pathway may be associated as well. A type II collagen mutation has been identified in Asian families with multiple members affected by AVN of the femoral head and no other detectable skeletal abnormalities. This gene mutation has not been identified in sporadic cases, however. There is an increased incidence of both major and minor birth defects in children with LP disease, suggesting that genetic or early developmental problems may be a factor.
Of the numerous possible environmental factors, subclinical trauma to the vessels supplying the femoral head seems to be the most likely cause. Several anatomic areas supplied by key vessels are especially vulnerable (as described earlier for AVN in general). Mechanisms can include traumatic compression of intraepiphyseal vessels or tamponade of extraepiphyseal vessels due to increased intraarticular pressure in the setting of joint effusions, but a universally applicable etiology has not been determined. In addition to arterial insufficiency, abnormal venous drainage has been demonstrated, and increased bone marrow pressures have been found in cases of osteonecrosis in adults. Regardless of the etiologic factors at play, infarction—perhaps multiple times—and subsequent repair lead to disease [48, 49].
Treatment
The treatment of LP disease invokes a great deal of controversy. Children who are less than 6 years old at onset are often not treated, regardless of the severity of femoral head involvement. Similarly, children of any age with minimal segmental necrosis may not be treated. Early in the course of disease, the goal of treatment is to prevent deformity of the femoral head by maintaining or improving its containment within the acetabulum, thus providing a template for cartilage shape. Improved alignment encourages normal development of the biologically plastic cartilaginous epiphysis while reducing pressure on the lateral segment of the epiphyseal cartilage. Containment strategies include ambulatory abduction bracing, varus femoral osteotomy, innominate osteotomy, and labral support shelf acetabuloplasty. Surgical intervention significantly improves the outcome in children with lateral pillar B hips who are older than 8—for those treated with surgery, 73 % will have spherical femoral heads (versus 44 % without surgery) [43]. However, early surgical intervention appears to neither prevent progression nor accelerate healing. Previous misguided attempts at surgical revascularization with bone grafting have led to premature fusion of the growth plate.
Late in the course of disease, treatment focuses on minimizing deformation of the femoral head caused by extrusion. If there is femoral head extrusion, hinged abduction may occur—the femoral head hinges instead of rotating within the acetabulum, resulting in a medial gap. This hinged abduction may be treated with excision of lateral head extrusion (cheilectomy or partial capitectomy) as a salvage procedure. In adolescents or young adults with healed LP disease, treatment depends on the specific causes of pain, dysfunction, or deformity. Gait abnormalities from femoral neck shortening and pain from femoroacetabular impingement (FAI) may need to be addressed surgically (see below for discussion of FAI, and see Chap. 27 for further discussion of surgical techniques and associated radiographic findings).
Imaging
Radiography
In general, LP disease is best imaged with radiographs, both for initial evaluation and for follow-up. Both frontal and frog lateral views of the hips and pelvis contribute useful information, but the lateral projection is almost always the most informative, since much of the resorption process is anterior. This area is superimposed on the more normal posterior epiphysis on the frontal view but is shown in profile on the lateral.
Examinations at 3–4-month intervals usually suffice, although in the later stages of repair, there is frequently little change between sequential studies and frequency of imaging may be reduced. Simultaneous evaluation of both hips helps track progress and also enables detection of asymptomatic bilateral disease. The usual gonadal shielding pertains.
Radiographic evaluation of other epiphyses (to investigate the possibility of other epiphyseal dysplasia, hematologic disease, etc.) is not advised unless unusual familial, age, or racial factors so indicate or if there is symmetric bilateral hip involvement that suggests multiple epiphyseal dysplasia. In case of questionable diagnosis, a bone age above the chronologic age may dictate further workup.
Subtle but definite radiographic abnormalities are commonly evident shortly after patients become symptomatic. Considering the time that must elapse between a vascular insult and the development of radiographic findings, it is likely that the vascular insult of LP disease is silent and that the blood supply has already been restored by the time of presentation.
Early Stage
Both height and transverse diameter of the affected ossific epiphysis are smaller than on the other side; diminished size implies that growth ceased or slowed several months earlier (Fig. 12.16). In addition, the distance between the inner margin of the acetabulum and the femoral metaphysis is larger on the abnormal side. Although this increase has been attributed to lateral displacement due to synovial swelling or effusion, arthrography generally does not demonstrate true subluxation, synovial hypertrophy, or synovial fluid under pressure. Rather, the acetabular cartilage is thickened, and there is overgrowth of the cartilage of the femoral head (see Fig. 12.14). These two radiolucent structures conspire to shift the observable epiphyseal ossification center laterally, although the medial joint space is not actually increased. Cartilage overgrowth along with the small size of the ossification center further indicates that the vascular insult or insults occurred long before the patient became symptomatic.
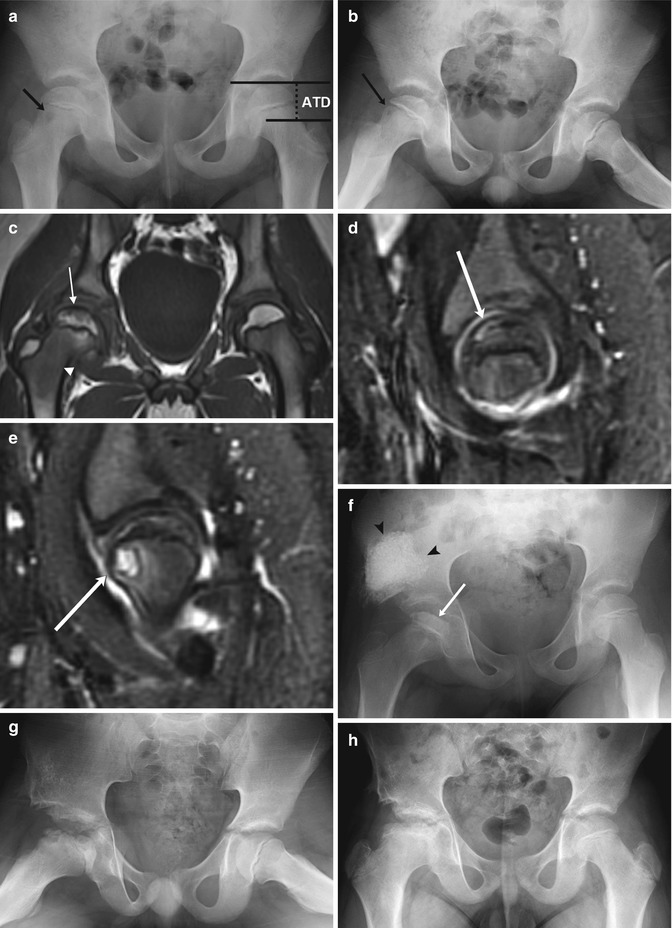

Fig. 12.16




LP disease in a 7-year-old boy with pain and limping. (a, b) There is mild loss of height of the right femoral epiphysis and a subtle lucency in the anterior aspect of the metaphysis (arrow). The articular-trochanteric distance is demonstrated on the normal left hip on image (a). (c) Coronal T1-W MRI shows decreased marrow signal in the subcortical femoral head (arrow) and neck (arrowhead). (d) Sagittal STIR image shows crescentic high signal in the anterosuperior aspect of the femoral head (arrow), representing a subchondral fracture. (e) Slightly more medial, another sagittal STIR image shows the metaphyseal lucency (arrow) with surrounding bone marrow edema and a joint effusion. Shelf acetabuloplasty was performed 3 months later, and after one more month (f) loss of height and sclerosis of the femoral head have progressed (arrow). Graft material appears dense and cloudlike (arrowheads). (g) One year later, femoral head fragmentation has progressed (graft is incorporated into iliac bone). (h) Four years later, after remodeling, the femoral neck is broad and short, and the femoral head sclerotic, wide, and flat. Note decreased distance between acetabulum and greater trochanter
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree