The pericardium is an important structure in the evaluation of patients with cardiovascular disease. Understanding pericardial anatomy and the complex hemodynamics associated with pericardial pathology is critical in assessing the pericardium and its impact on cardiovascular function. Unfortunately, clinical evaluation through history and physical examination, clinical laboratory tests, and electrocardiograms (ECG) may be nonspecific and overlap with other clinical syndromes in patients with pericardial disease. Advanced noninvasive imaging, including echocardiography, cardiac chest computed tomography (CT), and cardiovascular magnetic resonance (CMR), is vital in the evaluation and diagnosis of pericardial disease. When combined with a high degree of clinical suspicion, these modalities help advance our understanding pericardial pathophysiology and, ultimately, guide clinicians to optimal treatment and therapies.
Imaging Modalities
Several noninvasive imaging modalities are available to assess the pericardium, with each modality having its strengths and limitations. As a result of the complex nature of the pericardium and its impact on cardiovascular hemodynamics, multimodality imaging is often required to completely evaluate the pericardium.
Chest X-Ray
Chest x-radiography is often the first imaging modality that may suggest pericardial disease and prompt further testing. An enlarged cardiac silhouette may suggest the presence of a pericardial effusion, although this finding has limited sensitivity and poor specificity. Additionally, the chest x-ray (CXR) may detect pericardial calcifications and raise the possibility of constrictive pericarditis; however, calcification is not necessary for pericardial constriction and, in fact, is frequently absent in clinical presentations of constriction.
Echocardiography
Direct visualization of the heart through transthoracic echocardiography (TTE) is the most common noninvasive cardiovascular imaging modality for the evaluation of the heart and pericardium. Two-dimensional (2D) TTE with Doppler examination is an excellent, readily available, portable tool for the identification and assessment of pericardial disease. Real-time TTE can detect abnormal cardiac function and hemodynamic compromise secondary to underlying pericardial disease, including pericardial effusions and tamponade physiology. However, patient body habitus, chest trauma, and/or poor acoustic windows attributed to underlying lung disease (e.g., emphysema) may result in suboptimal views and limit TTE in visualizing the entire pericardium. Pericardial thickness is also not reliably assessed by TTE. Although transesophageal echocardiography (TEE) may be more accurate, it is moderately invasive, and the field of view remains limited to the available acoustic windows.
Computed Tomography
Cardiac CT and CMR provide a larger field of view than echocardiography, enabling visualization of the entire pericardium and the surrounding structures, providing excellent anatomic definition without limitation to specific windows. Although optimal imaging of the pericardium with CT and CMR requires ECG gating to minimize motion artifact, standard chest CT and nongated magnetic resonance imaging (MRI) performed for other indications often reveal most pericardial abnormalities, particularly pericardial effusions or calcifications. Cardiac CT and CMR may be useful when TTE findings are nondiagnostic, particularly for assessment of pericardial thickness and evaluation of pericardial constriction, loculated pericardial effusions, hematomas, and/or pericardial masses.
A specific advantage of CT (as compared with CMR) is its ability to identify pericardial calcification, a frequent, but not obligatory, finding in constrictive pericarditis. Cardiac CT with multidetector scanners and ECG synchronized data acquisition is now widely available, offering the advantage of very short scan times with limited cardiac and respiratory motion artifact. Additionally, CT provides excellent spatial resolution, as a result of the use of multidetector, high-resolution volumetric acquisition. Retrospective ECG-gated CT may also provide reasonable temporal resolution to assess interventricular septal motion in the evaluation of constrictive physiology, although this is better assessed by TTE or real-time CMR.
Disadvantages of CT include use of ionizing radiation, the need for intravenous iodinated contrast for adequate blood–tissue contrast, and limited hemodynamic assessment. Additionally, CT images may not adequately discriminate pericardial fluid from thickened pericardial tissue.
Cardiovascular Magnetic Resonance Imaging
CMR provides a comprehensive evaluation of the pericardium, both anatomically and hemodynamically, without ionizing radiation. CMR has the ability to obtain morphological, functional, and hemodynamic information in a single examination, which is frequently required when assessing pericardial disease and its impact on cardiovascular function. Balanced steady-state free precession (bSSFP) cine imaging is used to evaluate left ventricular (LV) and right ventricular (RV) motion and can assess the impact of pericardial disease on overall cardiac function. CMR also provides advantages over CT and echocardiography in its ability to characterize the contents of pericardial effusions and pericardial masses through the use of T1-weighted and T2-weighted imaging, first-pass perfusion, and late gadolinium enhancement (LGE). Additionally, real-time imaging by CMR may give vital hemodynamic assessments regarding cardiac motion and inflow patterns while the patient is free-breathing, allowing for the identification of constrictive and/or tamponade physiology. Multiplanar and multisequence CMR can be used to characterize the extent and composition of pericardial abnormalities and real-time CMR sequences allow for functional assessment, including chamber compression caused by ventricular interdependence and interventricular septal motion. Administration of conventional extracellular gadolinium (Gd)-based contrast agents may assist in detecting of pericardial inflammation or enhancing masses.
Normal Pericardial Anatomy
The pericardium is an elastic dual-layered fibroserous membrane that surrounds nearly the entire heart and extends superiorly to the origins of the great vessels. It consists of an outer fibrous component and a dual-layered inner serous sac. The tough fibrous outer pericardium is loosely attached to the sternum and costal cartilage (anteriorly) and the proximal great vessels (superiorly), and is more firmly attached to the central tendon of the diaphragm (inferiorly). The serous pericardium consists of an outer parietal layer, which is intimately adherent to the inside of the fibrous pericardium, and commonly referred to as the pericardium. The inner visceral serosal layer is adjacent to the heart (but not directly attached), covers the epicardial fat and vessels, and typically referred to as the epicardium. A film of clear pericardial fluid (~15–50 mL) normally separates the two serosal surfaces. The small amount of fluid minimizes the friction between the two layers as the heart expands and contracts. The serosal layers, consisting of the visceral and parietal pericardium, merge at two complex lines of reflection: one at the base of the aorta and pulmonary trunk, and the other at the insertions of the superior and inferior vena cavae and the four pulmonary veins. The entire pericardium generally lies between variable amounts of epicardial and pericardial adipose tissue.
The pericardial cavity is a complex space consisting of the pericardial cavity proper and interconnecting cul-de-sacs known as sinuses, which are further subdivided and connected to multiple recesses. Because of the merging of the serosal layers at two distinct points, the reflections of the serous pericardium become arranged as two complex tubes; one enclosing the base of the aorta and main pulmonary artery and the other enclosing the vena cavae and the four pulmonary veins. The transverse sinus is the space between these two pericardial tubes and lies superior to the left atrium (LA) and posterior to the ascending aorta and main pulmonary artery. The transverse sinus connects to the following four recesses: superior aortic, inferior aortic, left pulmonic, and right pulmonic. The cul-de-sac delineated by the inferior border of the tube, surrounding the venous structures, is the oblique sinus. The oblique sinus is an inverted U-shaped space which lies posteriorly to the LA and includes the posterior pericardial recess. The pericardial cavity proper includes the postcaval, left pulmonary venous, and right pulmonary venous recesses. The transverse sinus is the space between these two pericardial tubes and lies superior to the LA and posterior to the ascending aorta and main pulmonary artery. The transverse sinus connects to the following four recesses: superior aortic, inferior aortic, left pulmonic, and right pulmonic recesses. These recesses are usually linear in appearance and become more band-like and rounded as they accumulate with fluid. Appreciating the normal appearance of these sinuses and recesses is important because these spaces can be misinterpreted as adenopathy, dissection, or an abnormality of an adjacent mediastinal structure.
Although the pericardium is not required for normal cardiac function, it has several homeostatic roles. These include (1) limitation of intrathoracic cardiac displacement; (2) maintenance of normal ventricular compliance; (3) balancing RV and LV output over several cardiac cycles through diastolic and systolic interactions; (4) buffering of changes in chamber filling and output; (5) assisting atrial filling via more negative pericardial pressure during ventricular ejection; (6) limitation of acute dilatation; (7) minimizing friction between cardiac chambers and surrounding structures; and (8) providing an anatomic and immunologic barrier to inflammation and infection from contiguous structures such as the lungs. However, the presence of an intact pericardium may be detrimental in clinical situations in which fluid rapidly fills the pericardial space, resulting tamponade physiology.
Imaging Findings of Normal Anatomy
The normal pericardium is seen as a very thin linear density surrounding the heart. Discrimination of the pericardium from the myocardium by noninvasive imaging requires the presence of interposed epicardial fat or the presence of pericardial fluid with associated enlargement of the pericardial space. The thickness of the epicardial fat is increased in patients with obesity and/or diabetes and has been found to increase the risk of coronary atherosclerosis. The pericardium is best visualized over the right ventricle and, as a result of less epicardial and pericardial fat to provide adjacent tissue contrast, may not be well visualized around the left ventricle. Technical factors, such as spatial and temporal resolution, may affect pericardial thickness measurements because the normal pericardium is a thin irregularly shaped sac that moves with cardiac and respiratory motion, affecting the pericardial recesses to varying degrees. The superior aortic and left pulmonic recesses are less impacted by cardiac motion, whereas the inferior aortic recess, left pulmonary venous recess, and right pulmonic recess border the LA or left ventricle, and are often blurred because of cardiac motion.
The maximum normal pericardial thickness measured through advanced noninvasive imaging is typically 2 mm, which is slightly greater than normal cadaveric pericardial thickness, which measures up 1 mm. This difference is primarily caused by the inherent limitations of spatial and temporal resolution of CT and CMR. A possible pitfall in determining pericardial thickness by CT is that trace pericardial effusions, as well as partial volume effects, may produce the appearance of focal thickening. These imaging effects, along with normal intrinsic inhomogeneity in pericardial thickness, produce significant variations in pericardial thickness, and should not be mistaken for disease. The superior aortic recess of the transverse pericardial sinus, which surrounds the ascending aorta, may be mistaken for an aortic dissection or lymphadenopathy. The oblique pericardial sinus, which is located behind the LA, may simulate abnormalities in the esophagus, descending thoracic aorta, and subcarinal and bronchopulmonary lymph nodes. Recognizing the appearance of these normal structures is important to avoid mistaking them for mediastinal disease.
CMR, with its superior tissue contrast, is an excellent modality to evaluate the heart and pericardium. It is able to differentiate between small amounts of pericardial fluid and actual thickening. With CMR, the normal pericardium appears as a linear low-intensity signal between the high-intensity mediastinal and sub-epicardial fat. It is best visualized during systole ( Fig. 46.1 ). bSSFP cine imaging is used to evaluate cardiac motion and can be helpful in detecting pericardial effusions. Black-blood T1-weighted spin echo sequences, such as double inversion recovery fast spin echo, are commonly performed to evaluate the pericardial anatomy thickness and morphology. T2-weighted spin echo sequences, usually with a short tau inversion recovery sequence to null the signal from fat (referred to as a triple inversion spin echo sequence), is used to depict difference between pericardial thickening and small amounts of fluid. Normal pericardium will demonstrate low signal on both T1-weighted and T2-weighted sequences, whereas pericardial fluid will be high signal on the T2-weighted imaging. Pericardial inflammation may also demonstrate increased signal on T2-weighted images, but will commonly exhibit abnormal enhancement on the LGE imaging, helping to differentiate it from a small pericardial effusion. Real-time cine gradient echo imaging in the short axis during dynamic breathing allows for the detection of ventricular coupling in patients with suspected constrictive pericarditis. T1-weighted gradient echo myocardial tagged cine can be useful to detect the presence or absence of epicardial–pericardial slippage in cases with suspected fibrinous adhesions exist between pericardial layers. Phase contrast sequences using velocity encoded gradients can be used to assess for diastolic dysfunction and pulmonary venous flow patterns, which are abnormal in patients with restrictive physiology.

Pericardial Diseases
Pericardial Defects
Congenital pericardial defects are rare and uncommon. A spectrum of abnormalities exists, ranging from small defects in various locations to complete bilateral absence of the pericardium (extremely rare). The most common defect is an absence of the entire left side of the pericardium. Pericardial agenesis may be associated with congenital heart disease in a minority of cases, including atrial septal defect, patent ductus arteriosus, and tetralogy of Fallot. Although pericardial defects are generally found incidentally, smaller defects of the left pericardium may potentially cause herniation and entrapment of portions of the heart.
Imaging Findings of Pericardial Defects
CXR findings are nonspecific and may reveal evidence of cardiac displacement. Frontal CXR will demonstrate cardiac levorotation, with loss of the right heart border, and prominence of the pulmonary artery contour with complete absence of the left pericardium. Evaluation for pericardial defects on CT and CMR may be difficult because the normal pericardium is frequently not well visualized over portions of the heart, particularly along the left heart border. The diagnosis may be made on cross-sectional imaging by assessing for indirect signs of pericardial absence, including prominent cardiac displacement, interposition of lung tissue between the aorta and the main pulmonary artery, or between the inferior cardiac border and the diaphragm. Excessive LV apical motion on CMR cine images may also suggest the diagnosis.
Pericardial Cysts
A pericardial cyst is a congenital anomalous fluid collection arising from the pericardium and develops from persistent defective embryological pericardial remnants. They are usually unilocular, smooth, thin-walled structures filled with clear transudative fluid and may be attached with or without a peduncle to the pericardium. They are commonly found at the right anterior cardiophrenic angle but can be located throughout the mediastinum. Most patients are asymptomatic, with lesions detected incidentally on CXR or other chest imaging. However, they may be associated with chest pain, dyspnea, cough, or arrhythmias, likely as a result of compression of adjacent structures, and may require aspiration or removal.
Imaging Findings of Pericardial Cysts
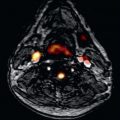
Pericardial cysts may present as a mass or abnormal contour adjacent to the cardiac silhouette on radiographic imaging. The location and incidental nature of the lesion will allow the imager to suspect the presence of this benign finding. Cross-sectional imaging of a suspected pericardial cyst will often distinguish it from a mediastinal tumor, hematoma, or loculated pericardial effusion. The CT and CMR appearance of the pericardial cyst is generally a nonenhancing, well-circumscribed, ovoid structure adjacent to the pericardium. The imaging characteristics of the contents are usually similar to water and will demonstrate low attenuation on CT, as well as low signal intensity on T1-weighted images, with homogeneous high intensity on T2-weighted images by CMR ( Fig. 46.2 ). A cyst may occasionally contain highly proteinaceous fluid that has high signal intensity on T1-weighted images. Intracystic septae may be observed, especially after the administration of contrast, but cyst contents do not enhance with administration of standard Gd-based contrast agents. Although typically benign, cysts often change size and shape with respiratory motion or body position.

Pericarditis
Pericarditis refers to any cause of pericardial inflammation, which may be local or systemic, and is the most common disorder involving the pericardium. The term pericarditis is a generalized term used for various forms of inflammation of the pericardium, with a spectrum of clinical presentations. These include (1) acute pericarditis without clinical evidence of a significant effusion; (2) pericardial inflammation with an effusion, but without major hemodynamic compromise; (3) cardiac tamponade, and (4) constrictive pericarditis. Categories of disease associated with pericardial disease are broad and include infection (e.g., bacterial or viral), radiation, autoimmune diseases, drug toxicity, inflammatory processes of contiguous structures (e.g., myocarditis and myocardial infarction), metabolic disorders (e.g., uremia, dialysis, myxedema), trauma (including iatrogenic causes), and neoplasms, as well as idiopathic etiologies. Most cases of infectious pericarditis are of viral causes. However, nearly any infectious disease (bacterial, fungal, parasitic, etc.) may involve the pericardium. In the Western hemisphere, most cases of acute pericarditis are idiopathic and presumed to be viral. Iatrogenic pericarditis from prior cardiac surgery or radiation is a major cause for pericarditis in the developed countries. Tuberculosis related pericarditis is still a common entity in developing countries. Signs and symptoms of acute pericarditis include chest pain (which is often pleuritic and positional in nature), a pericardial friction rub on auscultation, and widespread ST elevation on the electrocardiogram.
Imaging Findings of Pericarditis
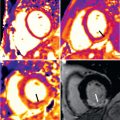
A pericardial effusion may be present with pericarditis, although its absence does not exclude the diagnosis, particularly in cases of acute pericarditis. CT or CMR may identify pericardial thickening, as well as pericardial effusions. However, differentiation between pericardial thickening and small effusions on echocardiography or CT may require further imaging. High intensity signal on T2-weighted imaging and pericardial enhancement after contrast administration by CMR suggest pericardial inflammation ( Fig. 46.3 ), which may be secondary to infection or neoplastic involvement. However, a lack of pericardial enhancement in the face of a medium or large pericardial effusion suggests a chronic, longstanding effusion. Metastatic disease or infection of the pericardium may also cause pericardial nodularity and thickening. CMR enables detection of pericardial thickening, effusion, and pericardial contrast enhancement.

Pericardial Effusion
Pericardial effusions are excess fluid accumulations in the pericardial sac. Accumulation may be attributed to a variety of causes, with most accumulations arising from the visceral pericardium. Types of effusions include simple fluid (either related to systemic fluid retention or underlying inflammation), hemopericardium, or rarely, chylopericardium. Hemopericardium may consist of either frank blood (e.g., direct trauma, iatrogenic perforation, or aortic dissection) or a bloody effusion (e.g., uremia or tuberculosis). Pericardial effusions related to a myocardial infarct may develop within days in response to transmural infarct, or later (1 week to several months) related to underlying inflammation, secondary to an immune response (Dressler syndrome). Similarly, pericardial effusions after cardiac surgery can be seen within days or later (1 week to 2 months, known as postpericardiotomy syndrome). Infectious or postinfectious pericardial effusions often resolve spontaneously but may continue as chronic relapsing pericarditis or progress to constrictive pericarditis. Myopericarditis occurs when an infectious or inflammatory process involves both the pericardium and the myocardium simultaneously. Malignant effusions are more commonly caused by metastatic disease or local invasion rather than primary neoplastic disease of the pericardium.
Purulent pericarditis is an infection of the pericardial space associated with purulent pericardial fluid. Fortunately, purulent pericarditis is less common since the advent of antibiotics. It may be caused by bacterial or fungal pathogens, with Staphylococcus aureus being the most common. When purulent pericardial infection is suspected, drainage, combined with intravenous antibiotic therapy, is generally required.
Cardiac tamponade results in life-threatening hemodynamic compromise via external compression of the heart by accumulating pericardial fluid. The accumulation of pericardial fluid surrounding the heart causes cardiac chamber compression leading to compromise of venous systemic return and cardiac output. It can be caused by pericardial disease of any etiology. Progression to cardiac tamponade is related to the following: (1) the rate of fluid accumulation in the pericardial space, (2) the ability of the pericardium to stretch (pericardial compliance), and (3) the ability to increase venous pressure to support right heart filling. Because the pericardium has limited short-term capacity to stretch, rapidly accumulating pericardial fluid collections (e.g., hemorrhage) can produce tamponade at relatively low volumes (~250 mL). On the other hand, more slowly accumulating effusions may be accommodated by pericardial stretching over time, as in the case of hypothyroidism. Large, slowly accumulating pericardial effusions (greater than 1–2 L) may be tolerated with little or no hemodynamic compromise. The symptoms of tamponade are nonspecific, so rapid diagnosis through clinical assessment and early imaging, typically by TTE, is required. Prompt treatment is typically required through drainage of the effusion, most commonly by pericardiocentesis. However, a surgical approach with a pericardial window/pericardiectomy may be indicated when the effusion is loculated, recurrent, or associated with a malignancy.
Imaging Findings of Pericardial Effusion
Pericardial effusions are frequently detected in subjects with suspected cardiac or pericardial disease, as well as incidentally when imaging is performed for other indications. On CT, common transudative effusions have attenuation similar to water, usually measuring less than 20 Hounsfield units. Attenuation higher than water suggests more complex, more proteinaceous fluid, as in malignancy, hemopericardium, purulent exudates, or myxedema. On CMR, transudative effusions will demonstrate water characteristics and will demonstrate high signal intensity on T2-weighted imaging and low signal on T1-weighted imaging. Transudative pericardial effusions are often even brighter than epicardial fat on gradient echo images. More proteinaceous effusions will tend to show intermediate signal intensity on both T1-weighted and T2-weighted images and lower signal intensity than blood in the ventricular cavities on bSSFP cine imaging ( Fig. 46.4 ). In hemorrhagic pericardial effusions, the appearance of blood will depend on the age of the collection. A fresh hematoma will appear homogenously bright on T1-weighted and T2-weighted images, whereas older collections show the effects of T1 and T2 shortening caused by methemoglobin formation and generally show heterogeneous signal intensity, with areas of high signal intensity on both T1-weighted and T2-weighted images. Chronic organized thrombi may have a dark rim and low signal intensity foci, which may represent areas of fibrosis, calcification, or hemosiderin deposition. The size of pericardial effusions can be readily assessed quantitatively by CMR using volumetric methods.