Diagnostic limitations exist in the assessment of postoperative nerve regeneration. This article describes the role of available methods, such as clinical assessment, electrophysiologic studies, and magnetic resonance neurography in the postoperative evaluation of peripheral nerve repairs.
Key points
- •
Nerve injuries may significantly affect patients’ life, and timely surgical treatment can improve outcomes.
- •
Diagnostic limitations exist with current methods of postoperative evaluation, and newer techniques that may play useful role in adequately following up nerve repairs are actively sought.
- •
Emerging imaging techniques, such as high-resolution magnetic resonance neurography may play an important role in the postoperative follow-up of nerve repairs.
- •
A multidisciplinary approach entailing active discussion of findings among radiologists, electrophysiologists, and peripheral nerve surgeons can be helpful in planning surgical treatment and follow-up of patients.
Introduction to peripheral nerve repair
Nerve injuries can significantly impair patients’ quality of life. Motor and sensory functional loss may result in substantial debilitation of these patients and may even have psychosocial repercussions. Thus, the physicians or surgeons dealing with such patients should have a good understanding of the principles behind optimal nerve injury management to enhance outcomes.
Although different types and indications of nerve repairs exist, the postoperative imaging of nerve repairs remains a new and thus poorly defined field. High-field (3-T) and high-resolution magnetic resonance neurography (MRN) imaging holds promise in both diagnostic and prognostic realms. In this article, the nerve injury grading, various types of nerve repair techniques, and approaches available for postoperative follow-up are discussed.
Introduction to peripheral nerve repair
Nerve injuries can significantly impair patients’ quality of life. Motor and sensory functional loss may result in substantial debilitation of these patients and may even have psychosocial repercussions. Thus, the physicians or surgeons dealing with such patients should have a good understanding of the principles behind optimal nerve injury management to enhance outcomes.
Although different types and indications of nerve repairs exist, the postoperative imaging of nerve repairs remains a new and thus poorly defined field. High-field (3-T) and high-resolution magnetic resonance neurography (MRN) imaging holds promise in both diagnostic and prognostic realms. In this article, the nerve injury grading, various types of nerve repair techniques, and approaches available for postoperative follow-up are discussed.
Peripheral nerve anatomy and pathophysiology
Knowledge of normal nerve anatomy is essential in understanding and managing peripheral nerve injuries; thus we briefly outline some pertinent anatomy here. Each nerve contains neural tissue and connective tissue. The basic unit of a nerve is the axon. A sheath of connective tissue called the endoneurium surrounds each axon. A bundle of axons are surrounded by the perineurium, forming a fascicle. The fascicles may convey pure motor, sensory, or autonomic functions, or a mixture of these. The connective tissue stroma that surrounds a group of fascicles is called the epineurium. The nerves can be injured by a variety of systemic causes or local mechanisms. The local injuries are typically divided into compression or penetrating injuries. Seddon and Sunderland have classified the basic management strategies of peripheral nerve injuries ( Table 1 ). Each degree corresponds to a more severe level of functional compromise that may increase the need for surgical exploration (eg, fourth-degree, fifth-degree, and sixth-degree injuries warrant surgical repair). Although diverse methods exist for treatment of these injuries, in general, several factors related to nerve injury or patient demographics influence the outcome of surgical treatment ( Table 2 ).
| Classification | Nerve Component Injured | Expected Recovery | Surgery Indicated | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Seddon | Sunderland Degree (Modified) | Myelin | Axon | Endoneurium | Perineurium | Epineurium | Extent | Rate | |
| Neurapraxia | First | Yes | No | No | No | No | Complete | Fast | None |
| Axonotmesis | Second | Yes | Yes | No | No | No | Good | Slow | None |
| Axonotmesis | Third | Yes | Yes | Yes | No | No | Variable | Slow | None or neurolysis |
| Axonotmesis | Fourth | Yes | Yes | Yes | Yes | No | None | None | Nerve repair |
| Neurotmesis | Fifth | Yes | Yes | Yes | Yes | Yes | None | None | Nerve repair |
| Sixth | Combination of injury | Variable | Variable | Variable | |||||
| Relevant Factors | Functional Outcomes |
|---|---|
| Patient’s age | Younger patient > Older patient |
| Level of injury | Distal > Proximal |
| Type of nerve injured | Pure nerves > Mixed nerves |
| Injured nerve | Radial nerve > Median nerve > Ulnar nerve |
| Nerves C5 and C6 and those of the upper trunk > Nerves C8 to T1 and those of the lower trunk | |
| Tibial nerve > Peroneal nerve | |
| Injury mechanism | Lacerations > Low-velocity gunshot injuries > High-velocity gunshot injuries |
| Transections > Crush or avulsion injuries | |
| Stretch injuries > Ruptures > Avulsions | |
| Time frame between injury and repair | The “earlier the better”, Waiting 2–3 weeks might be reasonable in some instances (blunt or ragged transections or injuries with significant crush or avulsion component) to allow time for better definition of the zone of injury |
| Type of repair | Neurolysis alone in lesions with (+) nerve action potential has good results 90% of the time |
| Direct end-to-end nerve repair > Interpositional nerve grafting | |
| Distal nerve transfer > Proximal nerve grafts | |
| Direct nerve transfers > Nerve transfers with interpositional grafts | |
| Cognitive capacity | Higher verbal learning and visuo-spatial logic correlates with improved sensibility outcomes |
| Early psychological stress | Early psychological stress hampers functional outcome and work resumption |
Types of nerve repairs
The available options for the treatment of peripheral nerve injuries include neurolysis, direct nerve repair, nerve graft, nerve transfer, tendon/muscle transfer, and bone/joint procedures.
Neurolysis
Neurolysis is a surgical procedure performed to release the injured nerve from the surrounding scar tissue. It is categorized into internal and external neurolysis. External neurolysis involves exposing the entire nerve diameter and releasing it from its sheath and surrounding scar tissue. Internal neurolysis implies excision and release of scar tissue between the nerve fascicles in the intraepineural space. External neurolysis is more commonly performed, because the results of internal neurolysis are not encouraging.
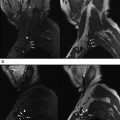
MRN can prudently guide the surgical treatment by identifying the perineural scarring, nerve hyperintensity, or entanglement by focal fibrosis or thickened fascia as well as internal heterogeneity of the nerve, reflecting scar tissue with irregular/disrupted fascicles, as commonly seen with neuroma in continuity ( Figs. 1 and 2 ).
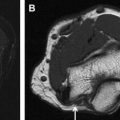
With successful neurolysis, the nerve T2 signal intensity should diminish over 4 to 6 weeks and size should become near normal. Failed neurolysis cases may show a combination of findings, such as worsening nerve T2 signal intensity, fascicular swelling, increasing nerve size, increasing perineural fibrosis, worsening regional muscle denervation changes, altered nerve course, or flattening due to encasement by fibrosis ( Fig. 3 A–C).
Direct Repair
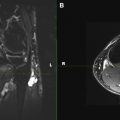
Most peripheral nerve surgeons pursue an end-to-end direct nerve repair whenever a tensionless coaptation is possible. This setting is ideal because tension between repaired ends can compromise recovery by interrupting vascular flow, subsequently leading to nerve scarring at both ends. Large nerve gaps (≥2 cm) can give rise to tension in anastomosis. This situation requires other alternatives, such as the use of nerve grafts. In our preliminary experience, MRN has proved useful to follow up nerve coaptations. The study should show homogeneous bright nerve signal intensity with current techniques, and over time, fascicular definitions should become perceptible as the axonal growth fills up the connections. The regional muscle denervation changes should also regress with time, observed as decreased signal intensity on fluid-sensitive fat-suppressed T2-weighted images and increased muscle bulk on T1-weighted images.
In surgical failures, the nerve signal changes from bright to black to bright at the site of coaptation as the nerve is followed on sequential scans from proximal to distal, because of interval scarring and formation of proximal neuroma ( Fig. 4 ). The regional muscle denervation changes also worsen, with progressive edema-like signal and atrophy.