Dina F. Caroline, Chandra Dass, Omar Agosto
The Stomach
Anatomy
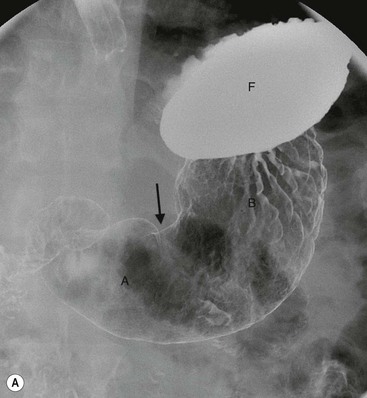
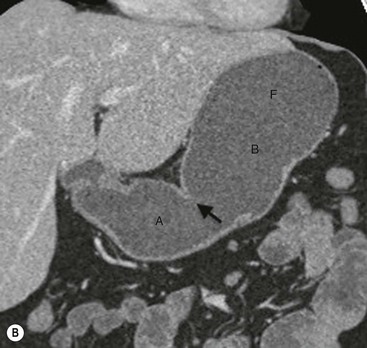
The oesophagus meets the stomach at the gastro-oesophageal (or oesophagogastric) junction (OGJ). The OGJ is formed by the lower oesophageal sphincter and the crural diaphragm, creating a barrier against acidic stomach contents. The stomach is divided into the cardia, fundus, body, antrum and pylorus. The medial and lateral curvatures of the stomach are known as the lesser and greater curvatures, respectively. The cardia connects the oesophagus to the stomach and lies close to the heart and left hemidiaphragm. The fundus is the rounded portion of the stomach that lies superior and posterior to the cardia. The body of the stomach lies distal to the cardia and comprises most of the organ. The body courses anteriorly and extends to the incisura angularis, which is an angle formed on the lesser curvature of the stomach. An imaginary line drawn from the incisura angularis perpendicular to the long axis of the stomach divides the body of the stomach from the distal third of the stomach or antrum. The pylorus with its short muscular cylindrical opening joins the stomach to the first part of the duodenum (Fig. 27-1). The antrum and pylorus course posteriorly to fix the second part of the duodenum retroperitoneally. Parietal cells, which produce hydrochloric acid, and chief cells, which produce pepsin precursors, are found in the fundus and body. Gastrin is produced in the antrum.
Histologically the stomach is composed of mucosa, submucosa, muscularis propria and serosa.1 The mucosa is composed of an epithelial layer with innumerable invaginations (pits or fovea) where the gastric glands are found. Loose connective tissue, lamina propria, is found between the gastric pits. A thin band of smooth muscle, the muscularis mucosa, forms the deepest layer of the mucosa. The submucosa consists of loose connective tissue and contains vascular and lymphatic channels, lymphoid follicles and autonomic nerves. The muscularis propria forms the major muscular structure of the stomach and is composed of inner oblique, middle circular and outer longitudinal layers. The circular muscle is thickened at the pylorus to form the pyloric sphincter. The muscle acts as a valve, regulating the outflow of gastric contents into the duodenum.
The serosa is the outermost layer of the stomach and is continuous with the gastrocolic (greater omentum), gastrosplenic and gastrohepatic (lesser omentum) ligaments.
Radiological Techniques
Fluoroscopy
Though barium examinations still represent the main radiological investigations for examination of the stomach in most parts of the world, they have been increasingly replaced by endoscopy and cross-sectional imaging. There are four basic components or techniques which can be utilised during the performance of these examinations: (A) double-contrast, (B) compression, (C) mucosal relief and (D) barium filling.2,3 Each has specific advantages as well as limitations in evaluating the stomach. Although it is rarely necessary to use all four of these components in a single examination, a thorough evaluation will typically utilise two or three of them. The single-contrast upper gastrointestinal examination emphasises compression, barium filling and mucosal relief, whereas the double-contrast examination utilises these techniques to a limited degree.
The double-contrast examination remains invaluable in the evaluation of the mucosal details of the stomach. Antiperistaltic agents are sometimes used to prevent motion artefacts in morphological imaging. The double-contrast technique involves the ingestion of an effervescent, gas-producing agent. The gastric walls are then coated with 100–150 mL of ingested high-density barium suspension. The patient is rolled through at least 360° to coat the mucosa of the entire stomach. Spot radiographs are then obtained in multiple projections (Fig. 27-2).
A properly performed single-contrast examination will emphasise mucosal relief, compression and barium filling. Images may be obtained with the inpatient prone, supine and/or upright positions. Mucosal relief radiographs are particularly obtained at the onset of the examination in both the prone and supine positions, with the objective being demonstration of the gastric mucosal fold pattern. Compression technique is also utilised.
A complete examination of the stomach will include several of the described techniques and include views to show duodenum and duodenal sweep filling to the ligament of Treitz. In most cases, elements of both the double-contrast and single-contrast study will be utilised in a biphasic or multiphasic examination.
Water-soluble contrast for an upper GI examination is reserved for evaluation of a suspected perforation, usually due to peptic ulceration or to demonstrate postoperative anastomotic leaks. Barium may lead to granuloma formation and peritoneal fibrosis if it enters the peritoneal cavity and should be avoided in these circumstances. Water-soluble contrast is innocuous in this situation as the contrast medium will be absorbed by the peritoneum and excreted by the kidneys. If possible the patient should be examined in both prone and supine positions to exclude a leak from the anterior wall.
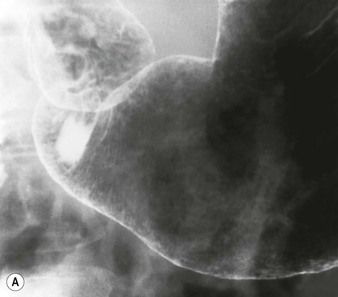
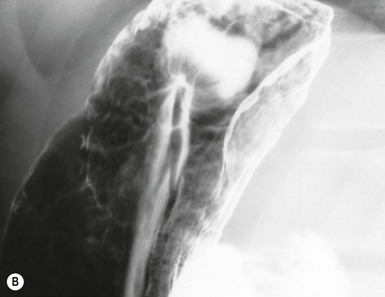
Gastric mucosa is characterised by two features, the areae gastricae, which form the mucosal surface pattern, and the gastric rugae, which form the gross or macromucosal pattern. The areae gastricae is composed of flat-polygonal tufts of mucosa separated by criss-crossing narrow grooves (sulci gastricae) which can be recognised in double-contrast studies as a reticular network of contrast filling the grooves between the mucosal tufts. The individual mucosal tufts normally have a diameter of 2–3 mm in the gastric antrum and 3–5 mm in the gastric body and fundus. Areae gastricae can be detected on double-contrast studies in nearly 70% of patients and are seen in greater frequency in the elderly.4 Visualisation of the areae gastricae is considered an indicator of adequate coating of the stomach.
Gastric rugae are smooth folds that tend to parallel the long axis of the stomach and are about 3–5 mm thick on barium studies. These comprise mucosa and a portion of submucosa. Abnormal rugal folds become thicker than normal and may be nodular. Contractions of the muscularis mucosa (the deepest layer of the mucosa) in the antrum may be seen as fine transverse folds in up to 10% of the population5 (Fig. 27-2C). In some cases these transverse folds, also known as gastric ‘striae’, may be seen in chronic antral gastrtis.
The fundus lies posteriorly in the left upper quadrant while the body and antrum course on a relatively horizontal plane to lie anteriorly and cross the midline. The distal antrum courses posteriorly to the right of the spine with the pylorus directed posteriorly. The greater curvature forms the anterior wall of the stomach and, similarly, the lesser curvature forms the posterior wall.6 Variability in the length of the mesenteric attachments leads to a more horizontal orientation of the stomach in muscular and obese people, and a more vertical orientation in slender people and in the geriatric population.
Cross-Sectional Imaging
Multidetector Computed Tomography (MDCT)
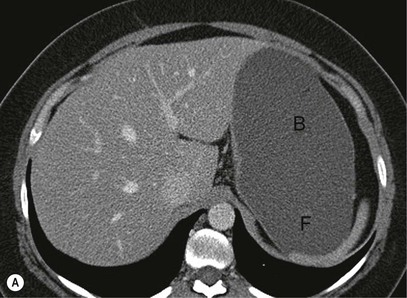
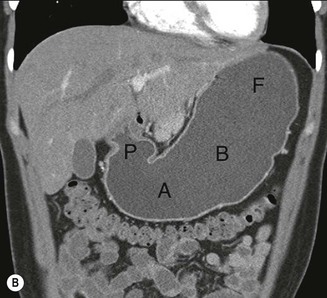
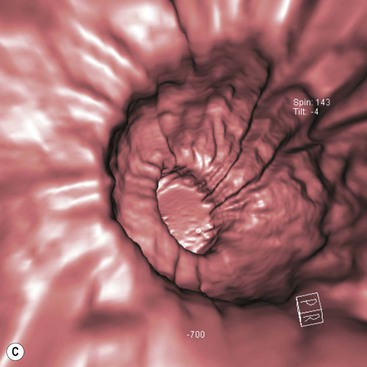
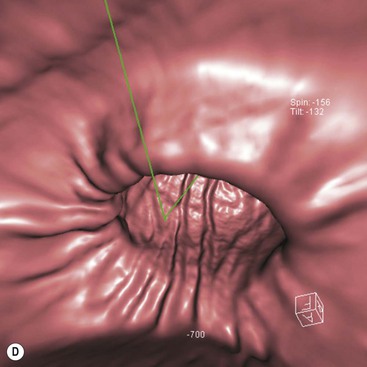
The role of CT for preoperative staging of gastric malignancy has been well established. Recent advances in MDCT with thin collimation offer the opportunity of near-isotropic imaging of the stomach in multiple planes, extending its role in the early detection of several gastric pathological processes. With proper distension of the stomach and optimally timed administration of intravenous contrast material, subtle luminal and mural lesions are evaluated with higher accuracy and a higher degree of confidence. Patient preparation includes no solid food for 6 h to empty the stomach. For dedicated CT evaluation of the stomach, stomach distension is achieved using negative contrast (6–8 g of effervescent gas-producing crystals with <10 cc water) or a neutral contrast (water, 750 mL approximately 15 min before imaging and an additional 250 mL table dose immediately prior to the study). Antiperistaltic drugs may be used to to reduce motion artefacts. In order to optimise visualisation with water distension, the patients with suspected lesion in the upper one-third of the stomach (gastric cardia or fundus) may be imaged in the supine/left lateral decubitus position and lesions in the distal two-thirds in prone/30° RPO position; with gas contrast, the positions are reversed. Post-intravenous contrast images of the upper abdomen with an imaging delay of 40–45 s (late arterial phase to assess gastric wall enhancement) followed by images of the chest, abdomen and pelvis at 70 s (portal venous phase to identify liver and nodal metastases) are typically obtained. Images are commonly analysed in multiplanar reconstruction mode (MPR) (Figs. 27-3A,B). Additionally, in the case of air contrast, surface-shaded volume-rendered images (similar to images obtained on the single-contrast barium study) or endoluminal fly-through view (virtual gastroscopy), or a combination of both may be used7,8 (Figs. 27-3C,D).
Based on its appearance in the arterial phase, the normal gastric wall is frequently described as single layered when only one high density layer is visualised or multilayered pattern, with a markedly enhanced inner mucosal layer, a middle hypoattenuating submucosal layer and an outer thin slightly higher attenuating muscular-serosal layer. Normal stomach wall thickness is a function of its degree of distension and normal values quoted in barium fluoroscopic literature are not applicable for CT gastrography. Optimal distension is critical for a successful CT gastrographic study.
Magnetic Resonance Imaging (MRI)
Dedicated MR imaging of stomach could be useful as an alternative for MDCT for locoregional staging of gastric malignancy. The patient preparation techniques are the same as for the CT and only water distension is used. Breath-hold fast T1W (2D fast gradient), T2W (2D fast spin echo) and post-gadolinium fat-suppressed T1W (3D fast gradient) images are routinely obtained. A trilaminar appearance of the normal gastric wall is well appreciated on T1W images, the middle layer representing submucosa with low T1 signal, compared to the inner wall (mucosa) and the outer wall (muscularis propria + serosa complex).9 Image analysis techniques are the same as described for MDCT. The enhancement characteristics of most stomach pathology are similar to that seen in contrast-enhanced CT.
FDG-PET and FDG-PET-CT
The use of FDG-PET in the tumour (T) staging of gastric cancer and identifying primary gastric lymphoma is limited due to the variable and usually significant background physiological uptake of FDG by stomach. FDG-PET may have a role in staging due to its potential to identify extragastric involvement in both gastric carcinoma and lymphoma. The imaging protocol is similar to that used in other oncological applications of PET-CT. Qualitative visual approach with or without semiquantitative parameters (standard uptake value) is used for image interpretation. Strict adherence to a consistent protocol is of paramount importance when PET-CT is used for monitoring response to therapy.
Gastric Pathology
Inflammatory Disease and Infiltrative Diseases
Helicobacter pylori and Diseases of the Stomach
Helicobacter pylori is a Gram-negative, flagellated, spiral bacterium that lives near the muscosal surface of the gastric mucosa. It is recognised as the strongest risk factor for the development of gastroduodenal ulcers. Studies have shown H. pylori infection in 60–80% of gastric ulcers and 95% of duodenal ulcers.10 Chronic active gastritis (superficial gastritis) is associated with H. pylori in close to 100% of cases. The organism was first isolated from gastric biopsies in the early 1980s by Marshall and Warren,11 and by the early 1990s H. pylori was firmly established as the causative agent for PUD.12 Helicobacter pylori is also considered by the World Health Organisation to be a dangerous carcinogen in the pathogenesis of gastric cancer13 and has been associated with MALT lymphoma (a low-grade B-cell lymphoma) of the stomach.14 In particular, MALT lymphoma has been associated with the H. pylori strains expressing the Cag-a protein.15
Before the demonstration of H. pylori as the causative agent for PUD, the stomach was believed to be a sterile environment because of its acidity. Helicobacter pylori was found to produce the enzyme urease, and as a consequence creates an alkaline ‘microenvironment’ for the organism in the gastric mucus, thus allowing it to survive in the stomach.16 Acute infection initially injures parietal cells, causing decreased gastric acid production. This is followed by the chronic stage of infestation, which is localised to the distal stomach and duodenum. Parietal cell function recovers, leading to abnormally high acid output. This causes antral gastritis and duodenitis, with approximately 1% of infected patients developing peptic ulcers every year.17 Many factors appear to determine whether or not a person infected with H. pylori will develop ulcers or other diseases and in reality the majority of patients infected do not develop any associated diseases. The precise mechanism of ulcer formation is not known; however, eradication of the organism by combined antibiotic and antisecretory therapy increases the healing rate of ulcers and lowers the recurrence rate.18
Helicobacter pylori is found worldwide but is much more prevalent in developing countries. In developed countries the infection is more common in the lower socioeconomic groups, among ethnic and racial minorities, and in immigrants from less developed countries. It is more common in older patients from all the above groups. The precise mechanism of transmission is not known but it is likely to be oral–oral and/or faecal–oral.19
There is about 90% prevalence of H. pylori in adenocarcinoma arising in the body or antrum of the stomach. The risk of developing polyps and gastric cancer is six times higher in patients with H. pylori infection and gastric cancer is much more prevalent in countries where the rate of H. pylori infection is high. A large meta-analysis conducted by Fuccio et al. demonstrated that the eradication of H. pylori in high-risk populations decreases the risk of gastric cancer.20 There is conflicting evidence regarding a possible association of H. pylori with non-ulcer dyspepsia19 (Table 27-1).
TABLE 27-1
Prevalence of Helicobacter pylori Infection with Upper GI Disease
| Disease | Prevalence (%) |
| Active chronic gastritis | 100 |
| Duodenal ulcer | 95 |
| Gastric cancer (body or antrum) | 80–95 |
| MALT lymphoma | 90 |
| Gastric ulcer | 60–80 |
| Non-ulcer dyspepsia | 35–60 |
| Asymptomatic population | 20–55 |
Gastric Ulcer
Gastric ulcers penetrate the stomach wall through the mucosa into the submucosa and frequently the muscularis propria. Gastric ulcer is a common gastrointestinal disorder and is amenable to reliable radiographic detection. Almost all gastric ulcers (95%) are benign, with about 70% now shown to be caused by H. pylori infection and most of the remainder due to NSAIDs and alcohol abuse. Gastric ulcers are most prevalent in the distal stomach and along the lesser curvature. They are more common on the posterior wall of the stomach than the anterior wall and least common in the fundus.21,22 Benign greater curvature ulcers found in the distal half of the stomach are most often associated with NSAID use. NSAID- and alcohol-related ulcers are most often seen on the greater curvature of the antrum, due at least in part to a direct toxic effect of the ingested material.23 Benign ulcers are much less common in the fundus and along the proximal half of the greater curvature. These should always be considered suspicious for malignancy. Ulcers may be multiple, and in such cases each should be evaluated independently for criteria of benignancy.
In the elderly, ulcers are more evenly distributed throughout the stomach, particularly proximally along the lesser curvature.24 Steroid use, hereditary factors, emotional stress and smoking are other factors that may play a contributory role in the development of gastric ulcers.
Radiographic fluoroscopic signs of gastric ulcers are described as to whether they are seen in profile or en-face (straight on). The location and size of the ulcer in the stomach and position of the patient during barium studies will determine which of the features will be shown in any specific case. Based on the radiographic features of an ulcer, a determination should be made as to whether it demonstrates benign, indeterminate or malignant characteristics. Ulcers with clear-cut benign features can be treated medically and followed to complete radiographic healing. Ulcers with indeterminate or malignant features must be evaluated endoscopically and biopsied. Most benign gastric ulcers detected on double-contrast barium studies are 5–10 mm in size. ‘Giant’ ulcers (>3 cm) are almost always benign but have a higher rate of complications of bleeding or perforation.
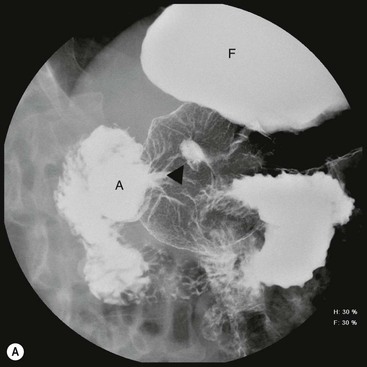
The en-face radiographic signs of gastric ulcers are best seen on double-contrast barium studies and to a lesser extent on compression views in the single-contrast examination. The primary sign of ulcer is a collection of barium in the ulcer crater along the dependent wall (Fig. 27-4A). Most benign ulcers are round or oval; some may have tear-drop or linear contour. If an ulcer is present on a non-dependent surface or is not filled with barium, it may be demonstrated as a ‘ring’ shadow, with barium coating the edge of the ulcer crater. If a ring shadow is seen with the patient in the supine position, and it is filled when the patient is turned prone, it is located on the anterior wall of the stomach. If the ulcer is on the posterior wall, it may be filled using the ‘flow’ technique—turning the patient with fluoroscopic guidance and observing while the barium bolus washes over the posterior wall.
En-face, a smooth mound of oedema is often seen surrounding the ulcer crater causing a circular filling defect. Radiating folds seen in healing ulcers should be smooth and symmetric and continue to the edge of the crater. The presence of normal areae gastricae extending to the ulcer crater is a sign of benignancy.
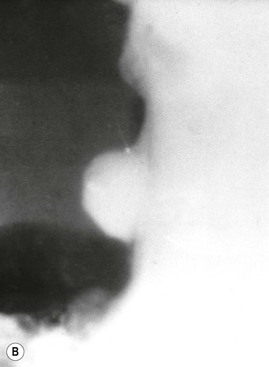
The classic description of a benign gastric ulcer refers to lesser curvature ulcers seen in profile. The ulcer, often referred to as the ‘ulcer niche’, projects beyond the lumen of the stomach.25 Sometimes a pencil-thin line of lucency, ‘Hampton’s line’, is present crossing the base of the ulcer26 (Fig. 27-4B). This is believed to represent preserved gastric mucosa with undermining of the more vulnerable submucosa. This sign is not common, but is virtually diagnostic of a benign ulcer. More often there is a thicker (2–4 mm) smooth rim of lucency at the base of the ulcer termed the ulcer collar. This is also a sign of benignancy. When there is more visible oedema associated with an ulcer, it forms an ulcer mound. The ulcer mound should be a symmetrical gently sloping mass (Table 27-2).
TABLE 27-2
Ulcers
| Findings | Benign | Malignant |
| Hampton’s line | Present | Absent |
| Extends beyond gastric wall | Yes | No |
| Folds | Smooth, even | Irregular, nodular, may fuse |
| Associated mass | Absent | Present |
| Carman meniscus | Absent | Present |
| Ulcer shape | Round, oval, linear | Irregular |
| Healing | Heals completely | Rarely heals |
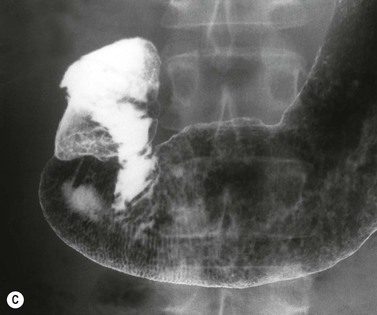
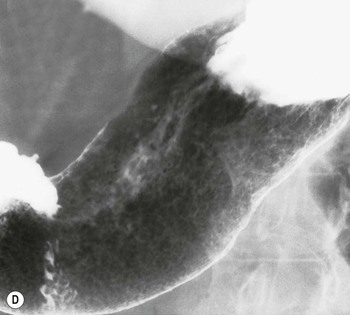
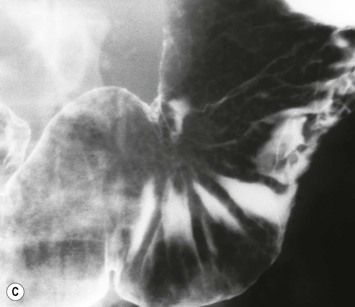
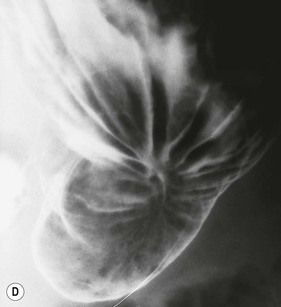
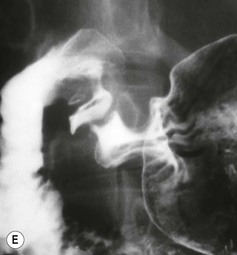
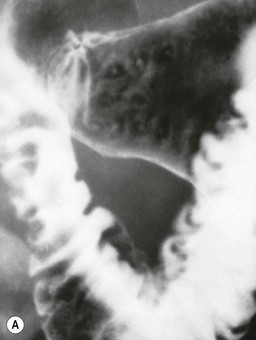
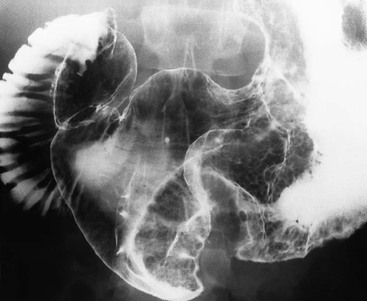
Almost all (>95%) benign gastric ulcers heal in about 8 weeks when treated medically.27 Benign ulcers may heal completely without any radiographic residua. As benign ulcers heal they may change shape from round or oval to linear crevices. There may be subtle retraction or stiffening of the affected wall (Fig. 27-4C). An easily recognisable radiographic sign of healed gastric ulcer is the presence of folds converging to the site of the healed ulcer. There may be a residual central pit or depression (Fig. 27-4D). The radiating folds should be uniform. Incomplete healing, irregularity of the folds, residual mass or loss of mucosal pattern all suggest the possibility of an underlying malignancy. The Carman meniscus sign is appreciated in the clinical setting of a large, flat ulcer with heaped-up edges. The edges of the ulcer trap a lenticular barium collection that is convex relative to the lumen when the edges are folded upon themselves during compression. These findings are indicative of a malignant gastric ulcer. Occasionally benign ulcers may heal with significant scarring. Severe retraction of the greater curvature from healed ulceration of the lesser curvature may cause narrowing of the mid-body of the stomach. Healing of antral ulcers may form prominent transverse folds or significant antral narrowing and deformity (Fig. 27-4E). Such scarring may lead to significant obstruction, an important complication of peptic ulcer disease (PUD).
The use of CT in the evaluation of gastric ulcers has been studied by several authors in recent years. Evaluation of the stomach by MDCT has been found to be useful in the detection and characterisation of benign and malignant gastric ulcers. The differences between malignant and benign ulcers are important in treatment planning and follow-up of patients with gastric ulcers. Evaluation by virtual gastroscopy (VG) and/or MPR has been studied and certain advantages and disadvantages have been elucidated.28
The benefits of VG include the ability to evaluate for mucosal changes which include morphological changes that are similar to conventional gastroscopy. Another benefit is that since optical endoscopic criteria have been well established for benign and malignant ulcers, then similar criteria can be applied to VG. Characteristics of malignant ulcers on VG include: ‘irregular or angulated shape; uneven base; asymmetric edge; bulbous enlargement; fusion or disruption of gastric folds reaching the crater edge’.28 Benign ulcer criteria by VG include: ‘smooth, regular, round or oval shape; even base; sharply demarcated or round edges; converging gastric folds with smooth tapering and radiation’.28
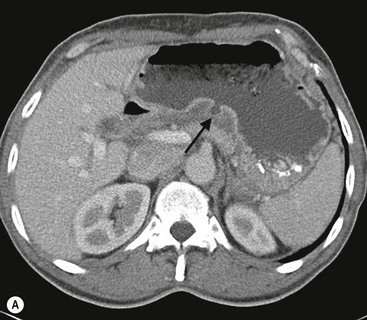
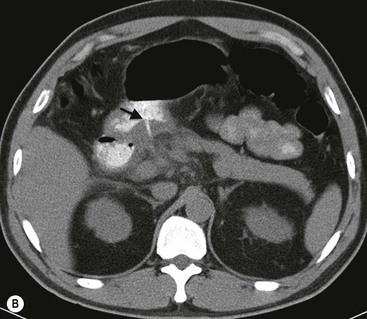
The use of MPR images allows for better visualisation of ulcers in multiple planes, facilitating better detection and characterisation of the ulcers. MPR also provides mural and extramural information, including, but not limited to, enhancement patterns of the gastric wall, lymphadenopathy and evaluation of adjacent organs. Characteristics of malignant ulcers on MPR images include: ‘strong enhancement of the wall at the site of ulcer greater than adjacent wall; marked periulcer wall thickening with loss of normal wall stratification; perigastric fat infiltration, lymphadenopathy or metastatic disease’.28 Characteristics of benign ulcers include: ‘no excessive enhancement when compared to adjacent gastric wall; mild periulcer wall thickening with preservation of wall stratification’28 (Fig. 27-5A). It is, however, important to understand that some of the features for benign and malignant lesions may overlap and differentiation of benign from malignant ulcers may not be determined by CT alone. MDCT evaluation remains complementary to conventional endoscopy (gastroscopy).29 Surgical complications like penetration and perforation are easily identified on MDCT using water-soluble contrast medium (Fig. 27-5B).
Gastric Erosions
Gastric erosions or aphthous ulcers are superficial ulcerations that do not penetrate the muscularis mucosa. They usually appear as small, shallow collections of barium 1–2 mm in diameter surrounded by a radiolucent rim of oedema. These are called ‘complete’ or ‘varioliform’ erosions (Fig. 27-6A). When the halo of oedema is lacking, the erosions are called ‘incomplete’ (Fig. 27-6B). These appear as short linear or serpentine lines or dots of barium. Gastric erosions are most easily detected radiographically when they are multiple and complete. They are most often detected on double-contrast barium studies but may also be seen with compression technique.30 Single or incomplete erosions are commonly detected endoscopically but are infrequently identified radiographically.31 Erosions heal without scarring. Gastric erosions are most often causally related to H. pylori infection.32 Other causes include alcohol, NSAID ingestion and Crohn’s disease. They may also be seen as a response to stress: for example, in patients with severe trauma.
Gastritis
Gastritis is a descriptive term with sometimes conflicting pathological, endoscopic and radiographic definitions. It is now better understood that many causes of gastritis, including H. pylori, alcohol and NSAID gastritis, lead to similar morphological changes.30 The most common findings are thick (>5 mm) folds with or without nodularity (Fig. 27-7). Erosions, while less commonly seen, are a frequent sign of H. pylori gastritis. Other signs of gastritis include antral narrowing, inflammatory polyps and prominent areae gastricae. The radiological findings are similar to endoscopic findings.
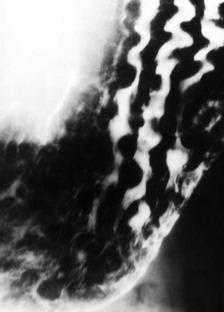
It is important to note that there is considerable overlap of findings between patients who are biopsy positive or negative for H. pylori. Therefore it is often not possible at this time to distinguish between ulcers and gastritis caused by H. pylori or those caused by chemical irritants (alcohol, NSAIDs and other aetiologies of gastritis).33 Owing to the prevalence of H. pylori, its association with many gastric diseases and effective treatment options, it is important for the radiologist to recognise findings that suggest the presence of the infection. It has been shown that thickened gastric folds, although nonspecific, is still the single most useful radiographic sign for the diagnosis of H. pylori gastritis and that the combination of thick folds and enlarged areae gastricae may be the most specific findings.34
Atrophic Gastritis
Atrophic gastritis is a combination of atrophy of the gastric glands with histological inflammatory changes. Atrophic gastritis is found in more than 90% of patients with pernicious anaemia and is characterised by loss of parietal and chief cells, leading to achlorhydria, and atrophy of the mucosa and mucosal glands.35 Atrophic gastritis causes a decrease in the production of intrinsic factor, which in turn causes malabsorption of vitamin B12.
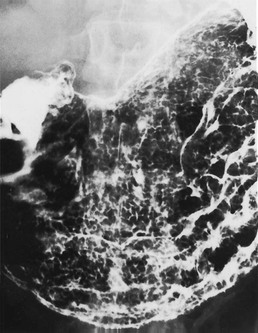
Radiographic findings of atrophic gastritis include loss of rugal folds and a tubular, featureless narrowed stomach (Fig. 27-8). Areae gastricae may be absent. The radiographic features are non-specific, but because of the important prognostic implications of atrophic gastritis, an appearance suggesting this diagnosis should trigger an appropriate clinical work-up.
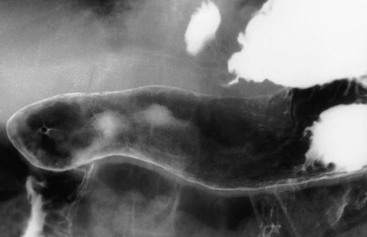
There is an association with gastric polyps and carcinoma36 and ulcers, both benign and malignant, may occur. Intestinal metaplasia, which may be seen histologically in atrophic gastritis, is considered a premalignant condition. The diagnosis of intestinal metaplasia may be suggested by areas of focal enlargement of the areae gastricae.37
Infectious Gastritis
Helicobacter pylori gastritis is by far the most common infection affecting the stomach. Tuberculosis, histoplasmosis and syphilis are usually lumped together with other granulomatous processes causing gastritis. Ulceration, thick folds and mucosal nodularity are common features, with antral narrowing being a late feature of these diseases. Monilia (Candida) may involve the stomach. This almost always occurs in the presence of severe oesophageal disease. Prominent aphthous ulceration may be seen in such cases.38
In immunocompromised patients, cytomegalovirus, crytosporidiosis and toxoplasmosis may occur. Radiographic findings are non-specific but there are some suggestive signs. Deep ulceration, and even fistulisation to adjacent structures may be seen with cytomegalovirus.39 Cryptosporidium primarily affects the small bowel, causing severe diarrhoea and thick, small bowel folds. It rarely involves the stomach but has been shown to cause deep ulcers, antral narrowing and rigidity.40,41 Strongyloides is a parasitic infection with a worldwide distribution affecting the stomach, duodenum and proximal small bowel evidenced by thickened, effaced folds and narrowing. In advanced cases, the stomach may be narrowed and have thickened folds.
Crohn’s and Other Granulomatous Diseases
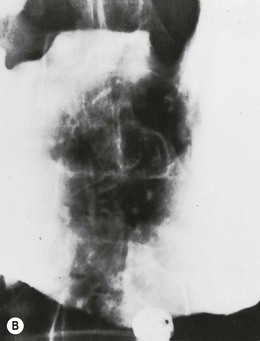
Granulomatous conditions of the stomach include Crohn’s disease, sarcoidosis, tuberculosis, syphilis and fungal diseases. Crohn’s disease is a chronic inflammatory bowel disease primarily affecting the ileum and colon. Gastroduodenal involvement occurs in up to 20% of cases, most often in the presence of ileocolitis.42 When the upper gastrointestinal tract is affected by Crohn’s disease, both the stomach and duodenum are commonly affected. However, involvement of the duodenum alone is more common than the stomach alone.43 A wide range of symptoms may be seen in patients with gastroduodenal Crohn’s disease; some are asymptomatic, others have symptoms more typical of PUD or with symptoms related to antral narrowing or gastric outlet obstruction. Diarrhoea caused by associated ileocolic disease is common. Gastrocolic fistula is an unusual complication. When it occurs, disease of the transverse colon extending to the stomach is most often the cause.44 Radiographic findings of gastric Crohn’s disease almost always demonstrate involvement of the antrum alone or antrum and body of the stomach.45 With early disease, findings include aphthous ulcers, larger discrete ulcers, thickened and distorted folds and sometimes a nodular (‘cobblestone’) mucosa (Fig. 27-9). These are indistinguishable from aphthous ulcers or erosions due to other causes. These findings represent the non-stenotic phase of Crohn’s disease. Stenotic disease caused by scarring and fibrosis can result in narrowing of the gastric antrum and pylorus into a funnel or ‘rams-horn’ shape, sometimes leading to foreshortening of the stomach which can be so severe that it simulates a partial gastrectomy. This scarred, funnel-shaped antroduodenal region may also be seen in other granulomatous diseases, including tuberculosis, syphilis, sarcoidosis and eosinophilic gastroenteritis. Antral narrowing may also mimic scirrhous gastric carcinoma.46
Hypertrophic Gastritis
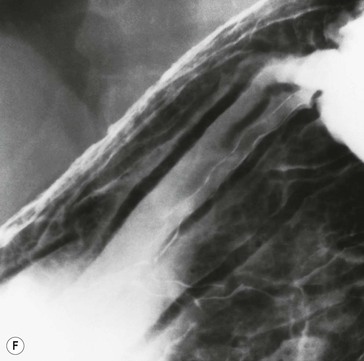
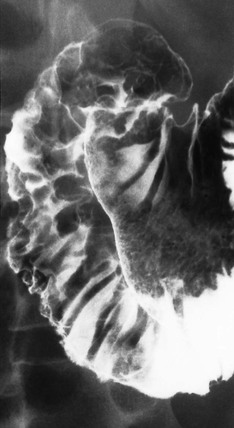
Hypertrophic gastritis is characterised radiographically by thickened folds, often greater than 10 mm in width, predominantly in the fundus and body, which are the acid-producing regions of the stomach. While the term is often used descriptively, the entity of hypertrophic gastritis is associated with glandular hyperplasia and increased acid secretion.47,48 Histologically, inflammation is not a prominent feature; thus, ‘gastritis’ is somewhat a misnomer. The areae gastricae pattern will become more prominent49 (Fig. 27-10). There is a high prevalence of duodenal and gastric ulcers in these patients. Many cases that had been previously classified as hypertrophic gastritis may in fact have been due to H. pylori infection. The differential diagnosis is primarily Ménétrier’s disease and lymphoma.
Ménétrier’s Disease
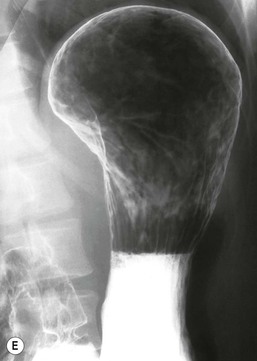
Ménétrier’s disease is a rare entity well known in the radiology literature because of its dramatic and characteristic appearance. This condition is characterised by hypertrophy of gastric glands, achlorhydria and hypoproteinaemia. Loss of protein from the hyperplastic mucosa into the gastric lumen results in a protein-losing enteropathy, and may produce disabling symptoms. The disease is characterised by markedly enlarged, often bizarre gastric folds most prominent in the proximal stomach and along the greater curvature.50 Radiographically, upper gastrointestinal or CT show massively thickened often lobular folds (Fig. 27-11). The folds remain pliable, which helps to differentiate it from carcinoma, where the stomach becomes rigid and aperistaltic. While in the classic description of Ménétrier’s disease the antrum is spared, it has been found to be involved in up to 50% of cases51 causing diffuse involvement of the stomach. Another feature of Ménétrier’s disease is the finding of increased fluid in the small bowel, which in turn may prevent optimal mucosal coating with barium.
Zollinger–Ellison Syndrome
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree