Fig. 4.1
Acoustic windows for thoracic sonography: 1. supraclavicular, 2. suprasternal, 3. parasternal, 4. transsternal, 5. intercostals, 6. subxyphoid, and 7. subdiaphragmatic
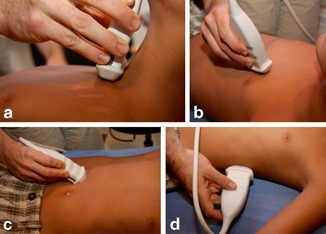
Fig. 4.2
Demonstration of select acoustic windows for assessment of the pediatric chest. a Suprasternal, b Parasternal, c Subxiphoid, and d Transdiaphragmatic. [2]
The Mediastinum
The contents of the mediastinum are organized into three compartments: anterior (thymus, vessels, lymphoid structures, and nerves), middle (trachea, mainstem bronchi, the heart and great vessels, and the hilar lymph nodes), and posterior (aorta, esophagus, and the sympathetic nerve chains). Particularly suitable sonographic windows to the mediastinum are the parasternal plane, suprasternal plane, and transsternal plane, especially in babies, as the bones are not yet well ossified, which allows for improved ultrasound access to the chest. The anterior-superior mediastinum up to the aortopulmonary window is easily visible via transjugular ultrasound examination. As a supplement, transesophageal and transbronchial ultrasonography may offer valuable information.
Anterior Mediastinum
Thymus
The thymus is the dominant structure within the upper pediatric chest and is critical in the development of the immune system. It is located in the anterior superior mediastinum and consists of two lobes that are fused in the midline. The size, shape, and imaging finding of the normal thymus changes with age. The thymus appears largest relative to patient size at birth and may extend into the neck or down to the cardiac apex. It increases in weight through puberty, achieving maximal weight between 12 and 19 years. After puberty, the thymus slowly involutes [7].
The thymus is easily accessible for ultrasound examination. The normal thymus has a triangular shape in the longitudinal section, while in cross section it generally shows a trapezoidal or horseshoe like shape (Fig. 4.3a and 4.3b). The thymus is located in the anterior to the great vessels; caudally it sits on the heart and sometimes extends to the diaphragm. Sonographically, a normal thymus has a homogeneous and reticular echotexture and is slightly less echogenic than the liver, spleen , and thyroid gland. It is hypovascular on Doppler imaging and has well-defined margins, as it is surrounded by a demarcating capsule. The abnormal sonographic thymus, therefore, will have an irregular or lobular margin, heterogeneous echogenicity, coarse echotexture, and calcifications [8].
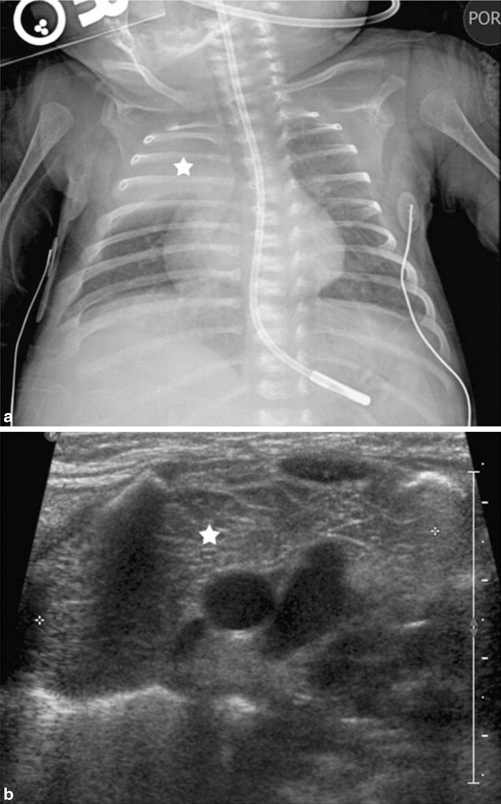
Fig. 4.3
Images of normal thymic tissue. a Chest radiograph demonstrates opacity in the right upper lobe (star). b Ultrasound reveals normal thymic tissue (star). [2]
Thymic Aplasia/Hypoplasia
Thymic aplasia is a condition where no thymic tissue can be detected due to underdevelopment or involution of the organ. The ultrasound examination is the diagnostic method of choice and is superior to the chest radiograph. A diminished thymic size is seen in infants and children during physiologic stress; however, most of the times it is a pathologic state. Etiology may be a primary congenital defect as in DiGeorge syndrome or ataxia telangiectasia, or may be a secondary to long-term glucocorticoid therapy or human immunodeficiency virus (HIV) [9].
Thymic Hyperplasia
Thymic hyperplasia is a disorder whereby there is an increased production of the normal thymic tissue. It is usually a benign process related to a stress situation or disease, for example, burns, other severe systemic illness, chemotherapy, or radiation therapy. It is important to distinguish this from thymic or other mediastinal mass or neoplasm, which ultrasound is able to do readily. Sonographically, the thymus maintains an echotexture and echogenicity which is identical compared to the normal thymus. The position of the thymus is normal in most cases, although the shape may be changed.
Thymic Masses
Primary thymic neoplasms in children are rare and usually incidental findings [10]. Thymomas occur in older children and adolescents who may present with paraneoplastic syndromes or myasthenia gravis [11]. These can be heterogeneous tumors with areas of necrosis and calcification (Fig. 4.4a, 4.4b, 4.4c), in contrast with thymolipomas which are homogeneously echogenic due to their high fatty content.
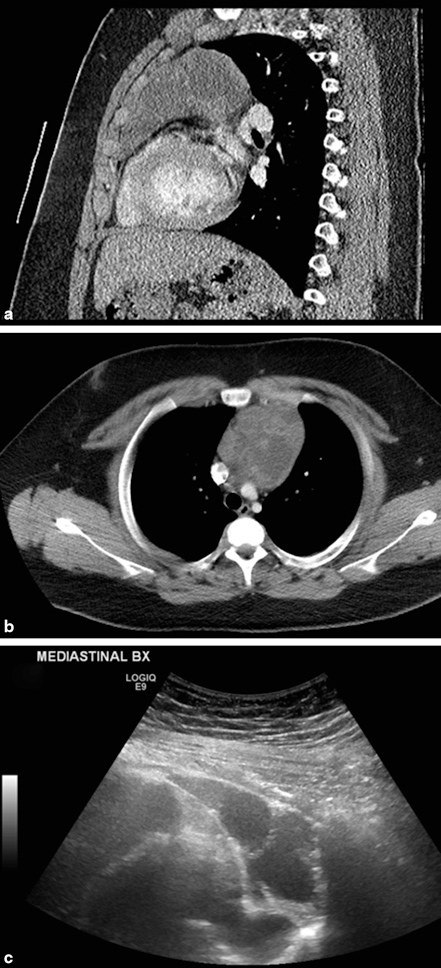
Fig. 4.4
Thymoma. a Ultrasonography shows a large mediastinal mass consisting of complex, septate, partly hyperechoic solid areas, and partly hypoechoic cystic areas. b and c CT scan confirms large mediastinal mass and heterogeneous nature
Secondary neoplastic thymic infiltration is more common and occurs with leukemia, lymphoma, and Langerhans cell histiocytosis. In these cases, the normal sonographic thymic pattern is replaced with variably echogenic and heterogeneous soft tissue and associated abnormal lobulation of the thymic capsule (Fig. 4.5). An infiltrated thymus loses its normal compliance and may be seen to displace and distort adjacent structures instead of conforming to their shape [9].
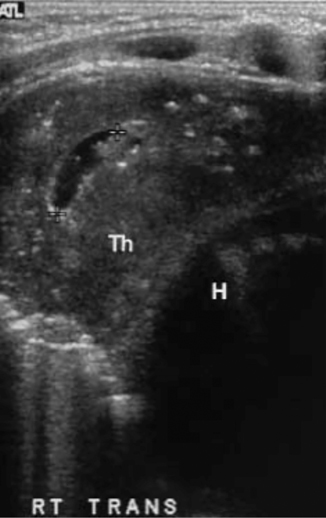
Fig. 4.5
Langerhans’ cell histiocytosis with thymic involvement. Transverse ultrasound scan demonstrates disruption of normal thymic anatomy with cystic area and strongly echogenic irregular foci, which proved to be calcifications on CT (Th—thymus, H—heart). [40]
Benign thymic cysts can arise from remnants of the thymopharyngeal ducts or result from degeneration of the thymus itself after mediastinal trauma or surgery. Most congenital cases of thymic cysts are diagnosed in childhood, presenting as slowly enlarging masses that may extend into the neck. Thymic cysts typically are unilocular with imperceptible walls and anechoic contents, though superimposed hemorrhage or infection produces cyst contents of variable echogenicity or even debris [8]. Sonographic demonstration of their continuity with the thymus allows diagnosis.
Lymphoma
The anterior mediastinum is a common site for neoplasms, in particular, lymphoma. The majority of children with lymphoma have anterior mediastinal involvement, more frequent with Hodgkin’s lymphoma than with non-Hodgkin lymphoma. Patients may present with constitutional symptoms such as fever or weight loss, respiratory complaints. Sonographically, lymphomas may appear as discrete masses, nodal enlargement (Fig. 4.6), or with diffuse thymic infiltration. They tend to be hypoechoic and hypovascular compared with inflammatory processes and other neoplasms [9].
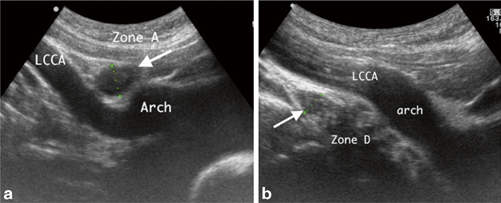
Fig. 4.6
Lymphadenopathy: Sagittal suprasternal ultrasound imaging in two children demonstrates lymphadenopathy. a A 3-year-old female lymphadenopathy (arrow) in zone A and b A 13-year-old male lymphadenopathy in zone D, which is echogenic in the center as compared to the echo-free vascular structures in recognized anatomical positions, that is, the aortic arch (Arch) and the left common carotid artery (LCCA). [41]
Germ Cell Tumor
Teratomas and other germ cell tumors may arise in the anterior (Fig. 4.7a, 4.7b, 4.7c) or posterior (Fig. 4.8) mediastinum. The ultrasound appearance of germ cell tumors is variable, ranging from purely soft tissue masses to heterogeneous masses containing fat, bone, and cystic elements. Tissue diagnosis is required before chemotherapy. Compression of the airways often associated with large anterior mediastinal masses is a contraindication for general anesthesia due to the danger of airway collapse [12]. Ultrasound-guided percutaneous biopsy is an excellent alternative in these patients and can be done safely under local anesthesia and mild sedation, even in critically ill patients.
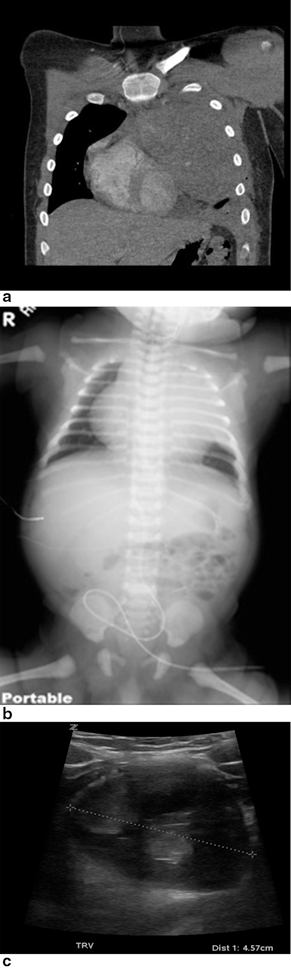
Fig. 4.7
Germ cell tumor, anterior mediastinal mixed germ cell. a CT scan and b chest radiograph demonstrate anterior mediastinal mass. Ultrasound c shows mass with mixed echogenicity, solid and cystic components
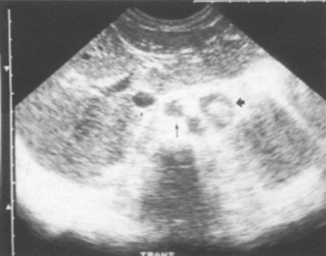
Fig. 4.8
Germ cell tumor, posterior mediastinal teratoma—42-mm wide mass of mixed echotexture in the posterior mediastinum displacing the inferior vena cave (arrow) and the aorta (arrow) anteriorly. [14]
Middle Mediastinum
Middle mediastinal lesions include cystic (bronchogenic, enteral duplication, pericardial, and lymphatic) and solid (lymphadenopathy) masses. Bronchogenic cysts are the most common intrathoracic cysts. They are thin-walled structures found around the carina that may compress or communicate with the trachea, resulting in collapse of a lobe. Esophageal duplication cysts may have a hypoechoic muscular rim typical of gastrointestinal duplications. Pericardial cysts have a typical appearance on plain radiographs and ultrasound can confirm their cystic nature. Lymphatic malformations are usually comprised of multiple loculated cysts with thin bands of intervening soft tissue. Normally hypovascular, lymphatic malformations may contain hemangiomatous components that demonstrate flow on color Doppler. Lymphatic malformations are frequently found in the vicinity of the great vessels and may cause compression of these vessels. Lymphadenopathy can arise from underlying neoplasia or infections, such as tuberculosis and fungal infections. Nodes appear abnormally enlarged and hypoechoic, often with hyperemia on color Doppler [2].
Posterior Mediastinum
Posterior mediastinal masses can often be best visualized via a posterior thoracic or paraspinal approach. Most of these are solid masses that arise from neural crest cells within the sympathetic ganglions. In order of decreasing malignancy, these include neuroblastoma, ganglioneuroblastoma, and ganglioneuroma [13]. The sonographic appearance of these tumors is nonspecific although calcifications can be seen; CT and MR imaging are more commonly used and more sensitive than ultrasound in this setting. Teratoma or other germ cell tumors can also be seen in the posterior mediastinum [14]. Less common are neurenteric cysts, hypoechoic thin-walled structures that have failed to separate from the neural canal during development [2].
Large Vessels
Vessels close to the heart are major arteries (aorta, pulmonary artery, and brachiocephalic trunk) and major veins (superior and inferior vena cava, internal jugular, and subclavian veins). Color Doppler ultrasound remains the principle method of investigation of vascular disease particularly within the subclavian and jugular vessels. Deep structures, such as the superior vena cava and the thoracic aorta, are difficult to evaluate sonographically in older pediatric patients and in these cases MR or CT angiography may be favored.
Vessel stenosis, aneurysms, and arteriovenous fistulae may occur from trauma, vascular access complications, or one of the arteritides. Diagnosis is made with color Doppler ultrasonography of the vessels. Arteriovenous fistulas demonstrate high diastolic arterial flow with elevated and turbulent venous flow. Stenoses demonstrate elevation of peak systolic flow through the narrowing, delayed systolic upstroke distal to the narrowing, and elevated diastolic flow due to downstream vasodilatation. Vascular malformations , intimal dissections, and other vascular anomalies can also be visualized directly by sonography.
The venous vessels are best visualized suprasternally. The most common indication for venous ultrasound is the evaluation of a suspected venous thrombosis. Acute thrombosis appears on ultrasound as hypoechoic material expanding the vessel lumen (Fig. 4.9a, 4.9a, 4.9c, 4.9d, 4.9e). Since compression of the subclavian and deeper thoracic veins is not possible, color Doppler investigation of both sides is helpful in uncovering subtle flow differences to identify proximal venous thrombosis.
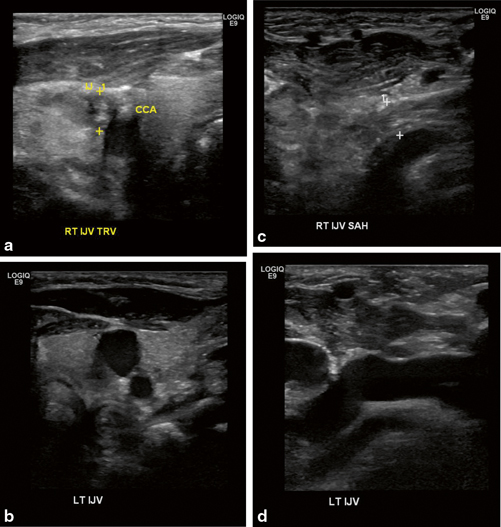
Fig. 4.9
Deep vein thrombosis of right internal jugular vein. Bilateral internal jugular veins are seen on sagittal (a and b) and transverse views (c and d)
Thoracic Outlet Syndrome
Thoracic outlet syndrome produces neurologic or vascular symptoms from compression of neurovascular structures in the upper chest. Anomalous cervical or first thoracic ribs, the anterior scalene muscle, and vascular variants may all contribute and may be seen by ultrasound. MR imaging provides exquisite anatomic detail of the thoracic outlet, but duplex ultrasound may provide important physiologic information by demonstrating alterations in arterial and/or venous flow, especially during reproduction of the position in which symptoms occur. Arterial flow may show acceleration or dampening of flow, depending on the proximity to the stenotic segment. Venous flow is more commonly affected, and there may be engorgement of the lateral subclavian and axillary veins and loss of transmitted cardiac waveforms [15]. Thrombosis may complicate repetitive venous compression, a typical finding in Paget–von-Schrötter syndrome, which is readily diagnosable by duplex ultrasound [16].
Chest Wall
The chest wall consists of skin, subcutaneous tissue, muscles, bone, and cartilage. Pathology involving these superficial structures is often clinically apparent and is easily evaluated with high-frequency linear ultrasound transducers.
Ultrasound can be used in the diagnosis of many infectious or inflammatory pathologies. Osteomyelitis is seen as fluid adjacent to bone signifying exudative reaction (Fig. 4.10). Cellulitis appears as diffusely increased echogenicity while defined fluid collections secondary to abscess can be diagnosed and drained under ultrasound guidance [17]. Rib fractures are identified by ultrasound in victims of non-accidental trauma by demonstrating disruption of the rib’s cortical surface as well as adjacent hematoma or callous formation depending on the age of the injury ([18], Fig. 4.11a and 4.11b). Traumatic separation of the costochondral cartilage from rib ends is visible sonographically but missed on plain radiographs.
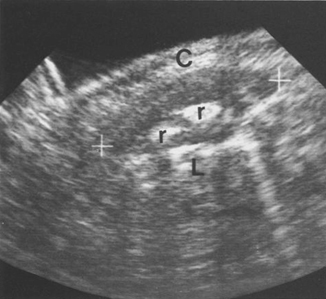
Fig. 4.10
Osteomyelitis. Longitudinal ultrasound scan of the right chest wall at the site of the soft tissue swelling. There is an obvious ovoid medium level echogenic structure around the ribs (r) indicating pericostal edema. C—chest wall, L—lung. [42]
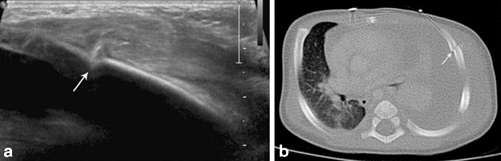
Fig. 4.11
Images in a 2-month-old boy with non-accidental trauma. Ultrasound a demonstrates a lateral left rib fracture (arrow) confirmed with CT b (arrow). [2]
Ultrasound reliably details the important variables for chest wall masses, including the location, size, contour, architecture, echographic pattern, compressibility, and relationship to other structures. Benign tumors, including hemangioma, lymphangioma, desmoid tumor, mesenchymal hamartoma, and lipoma, occur more commonly than malignant tumors [19]. Hemangioma can be diagnosed with a specificity of 98 % using the criteria of vessel density greater than 5 vessels/cm2 combined with a maximum systolic Doppler shift of greater than 2 kHz [20]. Lipomas are generally well-circumscribed echogenic masses usually located within the subcutaneous tissues. A lymph node can be identified by its echogenic fatty hilum containing the central nodal blood supply.
Other benign chest wall masses might not be easily diagnosed; tumors such as hemangioendotheliomas, tufted angiomas, and infantile myofibromatosis may share characteristics with hemagioma such that evaluation with CT, MRI, or biopsy is warranted for definitive diagnosis [8, 19, 21].
Venous malformations appear as a spongy, bluish deformable mass beneath the skin. Blood flow may be too slow to produce pulsed or color Doppler signal but with gentle compression and release the slow inflow of blood can be detected. Arteriovenous malformations are seen as jumbles of arteries and veins without associated mass. Lymphatic malformations have variably sized septated cystic components without flow on color Doppler most commonly found in the axillary region [22].
Malignant tumors of the chest wall most likely originate from bony structures, and may include Ewing sarcoma, rhabdomyosarcoma, and lymphoma [19]. Echogenicity of these malignant chest wall lesions is variable, and the margins may be distinct or infiltrative. Color Doppler flow of malignant chest wall lesions is usually increased. Chest wall and rib invasion can be detected as interruption of the normal muscular layers of the chest wall and loss of the normally smooth bony cortical surface. As with most other imaging, ultrasound is not histologically specific, and some benign lesions (such as abscesses and hematomas) may have aggressive sonographic appearances. Tissue sampling, often via ultrasound-guided biopsy, is usually needed for a definitive diagnosis.
Pleura
The healthy visceral and parietal pleura are poorly visualized on ultrasound examination, and evaluation of these structures relies on sonographic artifacts. For instance, the acoustic interface of the chest wall with normal aerated lung provides a strong reflective surface and produces a characteristic reverberation within the ultrasound image. This horizontal artifact is called an A-line and indicates the normal lung surface. The thin chest wall of infants and small children, however, may not demonstrate this artifact. In addition, aerated lung is also seen to move along the parietal pleural surface with respiration, termed the gliding sign [2].
Pleural Effusion
Ultrasound is able to demonstrate pleural effusions as small as 2–5 ml; therefore, it is much more sensitive in detecting pleural fluid than chest radiographs. The literature suggests that in most cases CT scan does not offer any advantage, and ultrasound may in fact be superior to CT for identifying debris or septated collections [23, 24] and in one study was associated with reduced need for VATS procedures and decreased readmission rates [25]. Ultrasound is a reasonable first-line modality for most patients with effusion , and CT scan should be reserved for complicated cases or preoperative planning.
The normal pleural space contains a tiny amount of fluid, but fluid is seen with ultrasound in less than 50 % of normal healthy children. Small effusions may be visualized better while the patient is in an upright position. This tends to collect the fluid in the posterior costophrenic recess. The most suitable transducer positions are the cranially angulated subxiphoid cross section and the longitudinal section of the middle and posterior axillary line.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree