The Urinary Bladder
▪ BENIGN BLADDER CONDITIONS
Filling Defects
Filling defects in the bladder must be differentiated from contour defects. A filling defect is an area of incomplete opacification on computed tomography (CT), excretory urography, or cystography and denotes something lying free within the bladder lumen. Examples include calculi, blood clots, and foreign bodies. Contour defects are mural or mucosal lesions that alter the contour of the contrast-filled bladder; examples include urothelial tumors, metaplastic and inflammatory masses, and wall thickening from any cause.
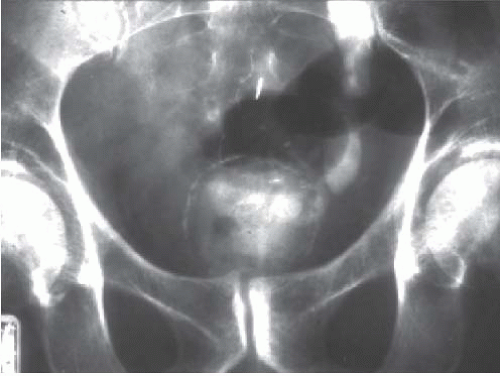
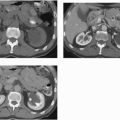
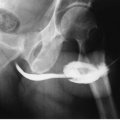
On excretory urography, a blood clot usually appears as an irregular, mobile filling defect, but occasionally may appear fixed in position and therefore may not be distinguishable from a tumor. Clots in the bladder may have a vermiform or wormlike appearance if bleeding has occurred in the upper urinary tract and a clot has formed in the ureter. Ultrasound shows that the blood clot is quite echogenic, unlike a bladder tumor, which often has an echogenicity similar to the bladder wall or other soft tissue. CT may help to distinguish a blood clot from a tumor by demonstrating the typical high attenuation of blood on enhanced images (60 to 80 Hounsfield units [HU]), and is useful to exclude a radiolucent stone. On occasion, a clot may be so large as to essentially fill the bladder (Fig. 15.1).
Foreign bodies within the bladder are most commonly inserted by the patient. Among other items, pens, pencils, matches, wire, tubing, and string can be seen within the bladder lumen. Foreign bodies may also be iatrogenic (e.g., broken or shorn catheter fragments) or may result from penetrating trauma (e.g., bullets). A rare complication of bladder catheterization is the introduction of pubic hairs into the bladder. These may become encrusted with calcium.
Edema
Mucosal edema around one ureteral orifice is most often caused by a stone impacted at the ureterovesical junction, but may also be due to the recent passage of such a stone. Focal mucosal edema may also result from bladder irritation by extravesical conditions such as acute appendicitis, Crohn disease, or sigmoid diverticulitis. Another cause of focal edema is an indwelling catheter, whose tip may irritate the bladder wall, typically near the dome. Generalized bullous edema may accompany acute cystitis secondary to infectious or inflammatory (irritative) etiologies.
Cystography shows the irregularity of the bladder wall produced by focal edema as a contour defect. Either ultrasound or CT may show an extravesical cause for localized bladder edema, such as an appendiceal abscess or other bowel disease. If no localized extravesical mass is seen, focal thickening may indicate a bladder tumor. On CT, the attenuation of the edematous bladder mucosa is only slightly less than that of soft tissue and presents as a contour defect of the bladder wall. CT is unlikely to distinguish edema from other causes of bladder wall thickening, including infiltrating bladder tumor.
Infectious Conditions
Virtually all acute infections of the bladder can, if severe, result in diffuse bullous edema of the urothelium, leading to a nodular irregular contour of the bladder on imaging studies. In many instances, this acute pattern will resolve in a matter of days and the bladder will return to its normal smooth appearance. However, severe infections can progress to a chronic phase, in which the bladder capacity is significantly reduced by fibrosis and contraction of the bladder wall. Identical appearing chronic cystitis occurs with many of the inflammatory (irritative) processes discussed later in this chapter.
Acute Bacterial Cystitis
Acute cystitis is present when more than 100,000 bacteria are present in 1 mL of urine. Most bacteria causing cystitis enter the bladder through the urethra. Escherichia coli is the most commonly encountered organism, but other common agents include species of Staphylococcus, Streptococcus, Proteus, Pseudomonas, Aerobacter, and Candida. Tuberculosis typically infects the bladder by descent down the ureter from a granulomatous focus in one or both kidneys.
Several factors contribute to a natural resistance of the bladder to infection. These include resistance of the bladder mucosa, the washing of organisms out of the bladder by normal voiding, trapping of organisms entering the bladder through the urethra by mucous secretion of the periurethral glands, and the bactericidal effect of prostatic secretions. Bladder infection is almost invariably the result of interference with one or more of these factors. For example, infection is more common when the bladder mucosa has been damaged by trauma, stone, or tumor; when outlet obstruction prevents bacteria from being completely washed out; and when bladder catheterization or instrumentation introduces infection by bypassing the protective mechanisms of the urethra and prostate. Acute cystitis can present with varying degrees of severity. In women, associated hemorrhage is common.
Acute cystitis usually responds well to antibiotic therapy and in uncomplicated cases does not progress to chronic disease. Although cystitis may recur two or three times a year in sexually active women, more frequent recurrence of acute cystitis and cases that are resistant to antibiotic therapy suggest an underlying cause. In such cases, imaging of the entire urinary tract and cystoscopic evaluation of the bladder are indicated to exclude causes such as urinary stone disease, bladder diverticulum, colovesical fistula, and perivesical abscess.
Cystitis due to tuberculosis is an interstitial process initially associated with mucosal edema and later progressing to bladder wall thickening and fibrotic contraction with reduced bladder capacity and a predisposition to vesicoureteral reflux. Rarely, there can be associated calcification of the bladder wall.
Emphysematous Cystitis
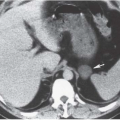
Emphysematous cystitis is a rare condition nearly always found in diabetic or immunocompromised patients. This is a true infectious cystitis most often due to E. coli, which ferments glucose to produce carbon dioxide and hydrogen. This gas is initially formed in the bladder wall and subsequently transgresses the mucosa into the lumen of the bladder. Emphysematous cystitis is associated with the same type of irritative symptoms as any other acute bladder infection. Gas within the lumen but not in the bladder wall is usually the result of catheterization or instrumentation in which air is introduced during the procedure. It may also be caused by a fistula between the bladder and the colon, small bowel, or vagina. Only rarely is such a finding caused by cystitis alone.
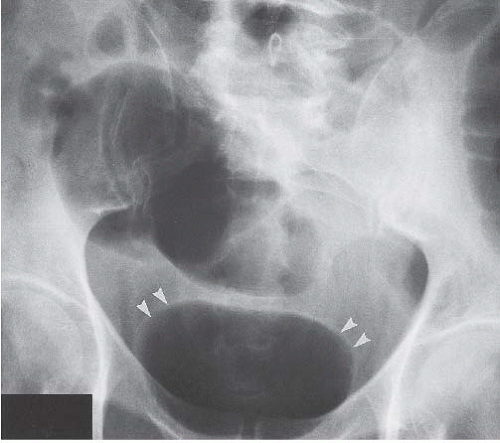
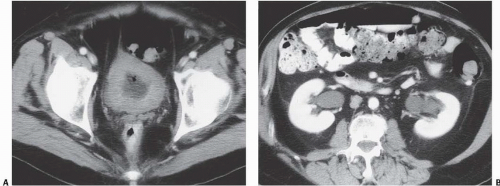
In patients with emphysematous cystitis, the plain film typically shows gas within the bladder and irregular streaky radiolucencies within the bladder wall (Fig. 15.2). Air within the bladder lumen should not be mistaken for rectal air. Air within the bladder conforms to the bladder shape, that is, ovoid with the long axis horizontal (like an egg on its side) and in central position low in the pelvis (Fig. 15.3). Gas within the rectum has a vertical orientation,
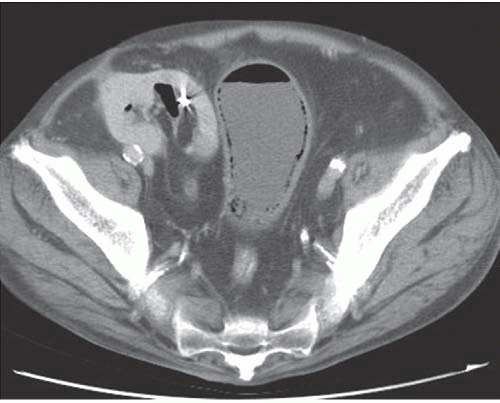
and the rectal folds often may be recognized. A lateral radiograph often distinguishes the anterior position of the bladder from the posterior location of the rectum. CT clearly shows the mural and luminal locations of the gas (Fig. 15.4).
and the rectal folds often may be recognized. A lateral radiograph often distinguishes the anterior position of the bladder from the posterior location of the rectum. CT clearly shows the mural and luminal locations of the gas (Fig. 15.4).
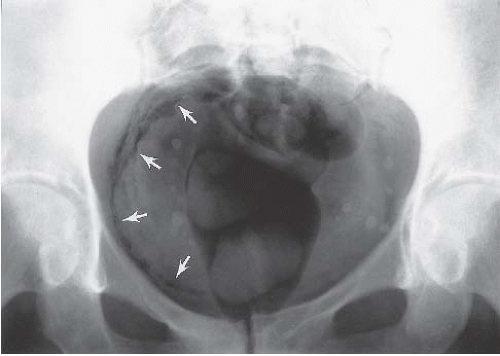 FIGURE 15.2. Emphysematous cystitis. Linear collections of gas within the bladder wall (arrows) indicate infection in this patient with diabetes mellitus. |
Emphysematous cystitis nearly always responds rapidly to appropriate antibiotic therapy and control of underlying diseases such as diabetes. It does not progress to a chronic condition.
Schistosomiasis
Schistosomiasis (Bilharzia) is one of the most common parasitic infections in the world, but is especially prevalent in the Nile Valley. Only Schistosoma hematobium affects the urinary tract. The definitive host is a human, and the intermediate host is a freshwater snail. Because the disease typically involves the intestines and bladder, eggs escape the human host in the feces and urine. These eggs, when deposited in fresh water, hatch into miricidia, which infest snails, develop, and emerge as cercariae. These cercariae infect humans directly through the skin, when humans are wading into or swimming in infested waters. After the skin is penetrated, they enter peripheral capillaries, drain to the lung, squeeze through the alveolar capillaries, and enter the systemic circulation. The cercariae that survive enter the mesenteric arteries and, hence, pass through the portal venous system. They develop into adolescent flukes in the portal blood and then migrate against the flow in the portal system using two suckers, which they alternately attach to the wall of a vein. They eventually reach the smallest venules in the wall of the bladder, probably through the hemorrhoidal plexus. From there, millions of eggs enter the urine, or are trapped in the bladder walls where they die, producing a severe granulomatous reaction. The granulomas calcify, causing linear streaks of calcium in the bladder wall. Large conglomerations of eggs in the bladder wall produce masses known as bilharzioma, which may also eventually calcify.
The clinical presentation of patients with schistosomiasis is typically hematuria. In initial stages, the bladder mucosa is edematous and hemorrhagic. Later, the bladder becomes fibrotic with a reduced volume and calcified wall. Cystoscopic examination is mandatory to exclude squamous cell carcinoma of the bladder, which has a markedly increased incidence in patients with schistosomiasis. Biopsy may be necessary to distinguish bilharzioma from carcinoma, although carcinoma usually presents as a later complication. Schistosomiasis rarely affects the kidneys or collecting systems, except by obstruction due to bladder disease or involvement of the distal ureter.
As the disease progresses, plain-film radiography may show dense calcification of the bladder wall or bladder stones (Fig. 15.5). Calcification of the distal ureter may also be seen, but is rarely present without bladder involvement. A bladder tumor should be suspected when follow-up studies show absence of wall calcification in areas that were previously calcified (Fig. 15.6). Although there are other causes of bladder calcification, including tuberculosis, radiation cystitis, alkaline-encrusted cystitis, and, rarely, bladder cancer, none is as common or as dramatic as schistosomiasis. The
ureterovesical junction may be partially obstructed by the changes in the bladder wall, resulting in ureteral dilation. Schistosomiasis may also affect the prostate and urethra. The disease is destructive and results in urethral fistula formation, producing a pattern similar to that seen with advanced tuberculosis. Fistulae may drain into the perineum, scrotum, suprapubic skin, or buttocks.
ureterovesical junction may be partially obstructed by the changes in the bladder wall, resulting in ureteral dilation. Schistosomiasis may also affect the prostate and urethra. The disease is destructive and results in urethral fistula formation, producing a pattern similar to that seen with advanced tuberculosis. Fistulae may drain into the perineum, scrotum, suprapubic skin, or buttocks.
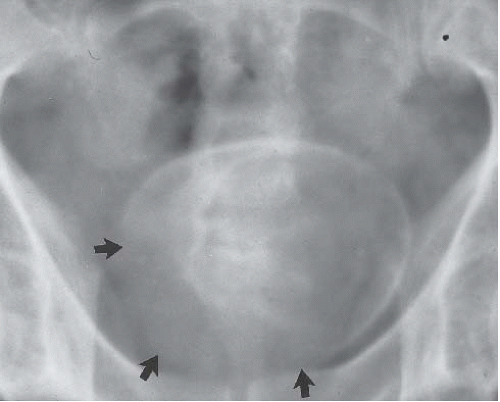
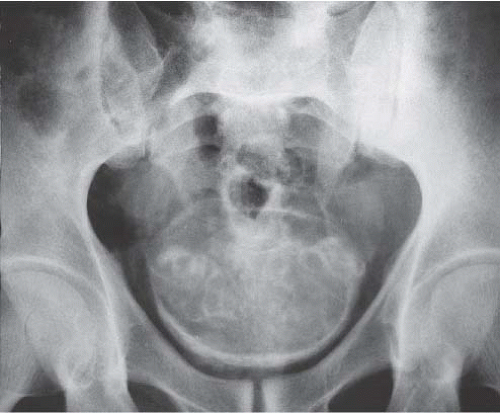 FIGURE 15.5. Plain-film radiograph of the pelvis showing marked bladder wall calcification. Seminal vesicle calcification is also seen through the bladder outline. (Courtesy of S. Sejeni, M.D.) |
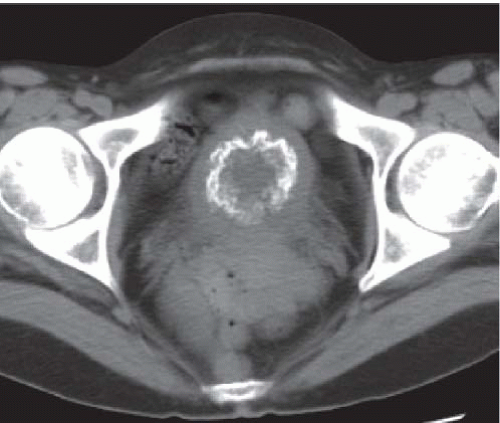
CT is more sensitive than plain radiographs in visualizing faint calcifications in the walls of the ureters and bladder in patients with suspected schistosomiasis (Fig. 15.7).
COMMON CAUSES OF BLADDER WALL CALCIFICATION
Schistosomiasis
Radiation cystitis
Alkaline encrustation cystitis
Tumor
Tuberculosis
Cytoxan cystitis
Amyloidosis
Candidiasis
Candidiasis of the bladder, typically seen in poorly controlled diabetic patients, causes fermentation of sugars in the urine, forming gas in the bladder lumen. It may also present as a fungus ball in the lumen. These fungus balls may be single or multiple and are seen as laminated, gas-containing filling defects within the bladder.
Inflammatory Noninfectious Conditions
Radiation Cystitis
Usually seen after external beam irradiation doses of 30 Gray (3,000 rads) or more, the acute form of radiation cystitis is associated with edema and hemorrhage. This acute phase is usually self-limiting and resolves completely. However, it may progress to mucosal ulceration, fibrosis, and a small-capacity bladder. Rarely, calcification of the bladder wall may occur. Hemorrhage in the acute phase may be severe, and angiographic embolization may be necessary to control bleeding. Cystography and CT show contour irregularity of the bladder wall due to edema that is indistinguishable from other causes of bladder mucosal edema. Over time, a fibrotic reaction may occur, resulting in chornic bladder wall thickening, reduced bladder capacity, and the appearance of calcification (Fig. 15.8). Fortunately, severe acute or chronic cystitis as a complication of radiation therapy is uncommon.
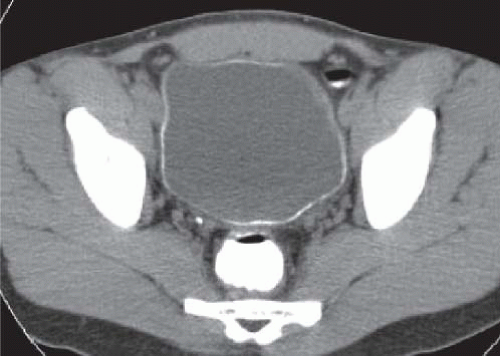 FIGURE 15.7. Schistosomiasis. Unenhanced axial CT image shows a thin rim of calcification in an otherwise normal appearing bladder wall. |
Hemorrhagic Cystitis
Many patients treated with the alkylating chemotherapeutic and immunosuppressive agents such as cyclophosphamide and ifosfamide have developed an acute hemorrhagic cystitis after treatment. The inflammatory change in the bladder is caused by exposure of the bladder mucosa to the metabolic end products of these agents (including acrolein), resulting in marked bladder edema with hemorrhage (Fig. 15.9). Massive bleeding has sometimes occurred and has required aggressive measures for control, including angiographic embolization or cystectomy.
The acute form of hemorrhagic cystitis, usually after high-dose intravenous injections of cyclophosphamide and ifosfamide, has tended to resolve with symptomatic therapy with no adverse sequelae; however, a chronic form sometimes may occur after many months of oral cyclophosphamide therapy, progressing to a contracted bladder. Bladder wall calcification is a rare finding in these patients (Fig 15.10). There is also an increased incidence of bladder carcinoma in patients being treated with cyclophosphamide (Fig. 15.11) or ifosfamide.
In recent years, chemoprotective agents have been utilized in patients treated with alkylinating chemotherapeutic agents, with one of these, mesna, having been observed to reduce the incidence of hematuria by binding to and inactivating acrolein (via sulfhydryl groups in the mesna molecule). Administration of mesna may also result in a decrease in the likelihood of subsequent development of bladder cancer, although data here are still lacking.
Eosinophilic Cystitis
Eosinophilic cystitis occurs in patients with severe allergic conditions and is more common in women. Eosinophilic infiltration of the bladder mucosa and submucosa results in edema, hemorrhage, and ulceration. Imaging of the bladder shows mucosal irregularity associated with focal or diffuse wall thickening. The condition usually responds well to steroids.
Alkaline Encrustation Cystitis
Alkaline encrustation cystitis may occur in association with bladder infection, typically with Proteus mirabilis. Urea-splitting bacteria may cause a virulent cystitis, resulting in areas of necrosis and sloughing of the mucosa and a severe inflammatory reaction in the muscularis and adventitia. An alkaline urine is produced by the release of ammonia from urea, which leads to calcium salt deposition in the necrotic areas of the mucosa. Calcification may then be seen in the bladder wall (Fig. 15.12).
Interstitial Cystitis
Interstitial cystitis is seen only in women, usually after menopause. The mucosa is hemorrhagic, and a typical finding at cystoscopy is ulceration near the bladder dome that cracks and bleeds with bladder distention (Hunner ulcer). Infiltration of the bladder wall with chronic inflammatory cells results in fibrosis and a very small bladder, often with a capacity of no more than 30 to 50 mL (Fig. 15.13). Interstitial cystitis has no known etiology, and no associated conditions have been identified. The condition is extremely debilitating because of the small bladder capacity and the pain associated with overfilling.
Malacoplakia
Malacoplakia of the bladder is of unknown etiology, but is associated with conditions such as pulmonary tuberculosis, chronic osteomyelitis, and long-standing malignant disease elsewhere in the body. The bladder mucosa is involved with multiple, yellow-gray plaques that tend to occur in the bladder base. The histology of these plaques shows histiocytes, lymphocytes, and plasma cells. Michaelis-Gutmann bodies in the biopsy specimen are diagnostic and are thought to result from phagocytized bacteria. Radiographically, rounded contour defects predominantly in the region of the bladder trigone may be difficult to differentiate from cystitis cystica or cystitis glandularis. Occasionally, this benign process may resemble a tumor, requiring biopsy for diagnosis.
Uncommon Inflammatory Noninfectious Conditions
Amyloidosis
Amyloidosis of the bladder is extremely rare. It may be primary or secondary and may be associated with amyloidosis elsewhere. Affected patients present with hematuria and urinary frequency.
Cystoscopy shows irregular infiltrating lesions in the mucosa and submucosa that bleed readily. Biopsy is necessary for diagnosis. Excretory urography, ultrasound, and CT may show multiple contour defects projecting into the bladder from the bladder wall, but the appearance is nonspecific and cannot be distinguished from other mucosal bladder lesions. The bladder base is often spared. Rarely, bladder wall calcification develops within the submucosal infiltrations.
Cystoscopy shows irregular infiltrating lesions in the mucosa and submucosa that bleed readily. Biopsy is necessary for diagnosis. Excretory urography, ultrasound, and CT may show multiple contour defects projecting into the bladder from the bladder wall, but the appearance is nonspecific and cannot be distinguished from other mucosal bladder lesions. The bladder base is often spared. Rarely, bladder wall calcification develops within the submucosal infiltrations.
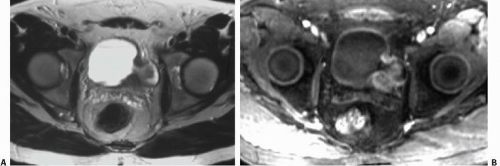
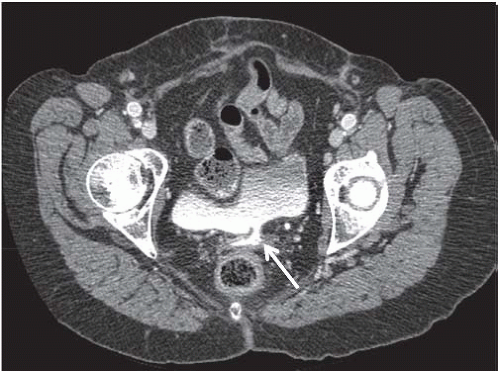
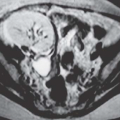
Endometriosis
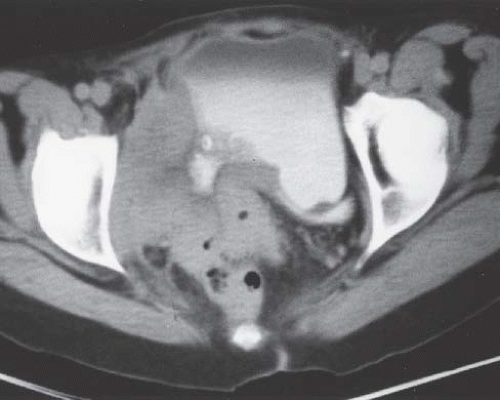
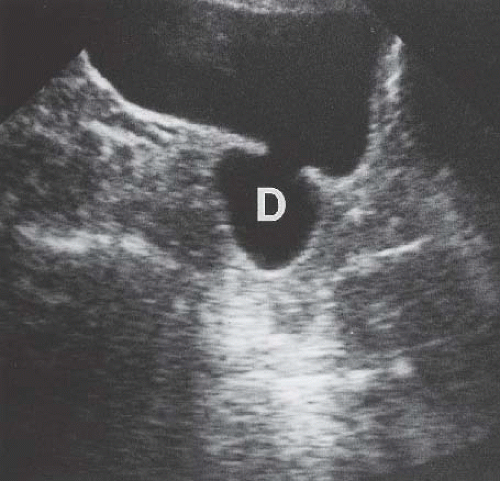
Although uncommon, the bladder is the most frequent site in the urinary tract to be involved with endometriosis. Endometrial tissue may infiltrate through the bladder muscle and produce mural masses projecting into the bladder lumen. This condition results in cyclic hematuria that is more prominent at menstruation. Ultrasound, CT, and MRI may be helpful in showing a mural mass protruding into the bladder, typically near the dome (blue dome cyst), as a direct extension of an extrauterine endometrial mass (Fig. 15.14).
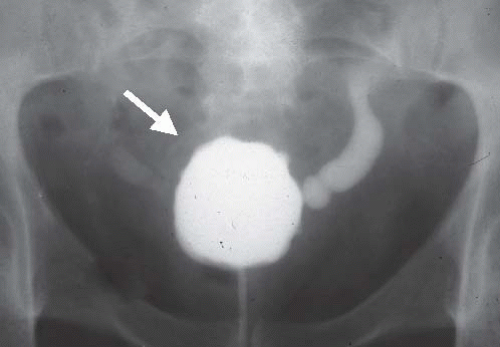 FIGURE 15.13. Interstitial cystitis. Voiding cystourethrogram shows small capacity bladder with bilateral reflux and thickened wall (arrow). The patient could not tolerate further bladder distension. |
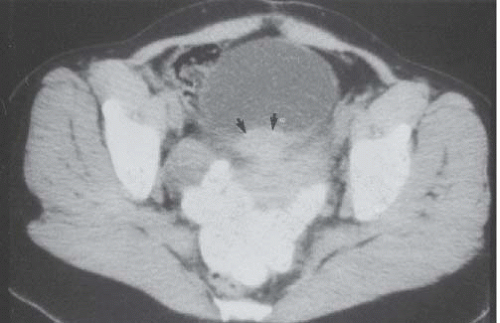 FIGURE 15.14. Endometriosis of the bladder. Contrast-enhanced axial CT image shows a soft-tissue mass involving the bladder wall (arrows) and extending directly from an irregular pelvic mass. This proved to be endometriosis. (From Amis ES Jr, Newhouse JH. Essentials of Uroradiology. Boston, MA: Little, Brown and Company; 1991:297, with permission.) See Fig. 15.7 for related excretory urogram. |
Perivesical Infectious and Noninfectious Inflammatory Lesions
Inflammatory lesions in the pelvis can abut the bladder and produce reactive thickening of the bladder wall (Fig. 15.15) and mucosal irregularities. Mucosal changes may at times be so severe as to appear tumefactive. If untreated, fistulization can occur. Typical conditions that can involve the bladder include appendiceal, diverticular, and tubo-ovarian abscesses. The bladder changes caused by such conditions may at times be difficult to differentiate from metaplastic processes or urothelial neoplasms originating in the bladder. Direct extension of tumor into the bladder from adjacent organs also can have a similar imaging appearance. However, the clinical presentation of a perivesical abscess should suggest an infectious process.
Proliferative/Metaplastic Conditions
Cystitis Cystica
Cystitis cystica is a benign condition, most commonly found in women with recurrent or chronic cystitis secondary to E. coli infection. Cystic lesions 1 to 2 cm in diameter tend to occur in the
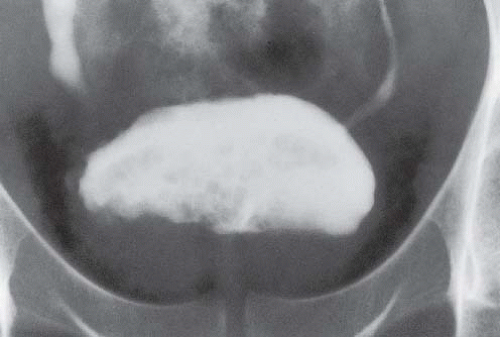
bladder base and on the trigone. These lesions result from degeneration of subepithelial clusters of transitional cells known as von Brunn nests. Radiographically, the bladder base shows multiple, rounded contour defects (Fig. 15.16), a finding suggestive of, but not specific for, cystitis cystica.
bladder base and on the trigone. These lesions result from degeneration of subepithelial clusters of transitional cells known as von Brunn nests. Radiographically, the bladder base shows multiple, rounded contour defects (Fig. 15.16), a finding suggestive of, but not specific for, cystitis cystica.
Cystitis Glandularis
Cystitis glandularis occurs with further metaplasia of the von Brunn nests into glandular structures resembling intestinal epithelium. It is seen with chronic or recurrent infections. These lesions are considered premalignant, as they may occasionally transform into adenocarcinomas. On cystoscopy, there are typically one or more irregular mucosal lesions grossly resembling bladder cancer. Radiographically, these lesions cannot be clearly differentiated from cancer (Fig. 15.17) and often require biopsy to establish the diagnosis.
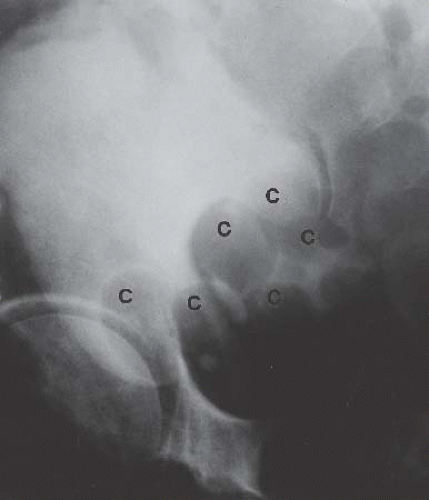 FIGURE 15.16. Cystitis cystica. Bladder phase of an excretory urogram (oblique view) shows smooth rounded contour defects in the bladder base (C) representing cysts. |
Nephrogenic Adenoma
Nephrogenic adenoma is a very rare proliferative response of the bladder epithelium to chronic infection or irritation. The name derives from the histologic appearance of the lesions, which is similar to that of the proximal tubules of the nephron. This lesion is more common in the bladder than in other locations in the urinary tract. The lesions range from mucosal irregularities to large malignantappearing masses arising from the bladder wall (Fig. 15.18). No specific findings allow for differentiation of nephrogenic adenoma from bladder cancer, and biopsy is necessary to establish the diagnosis. Although this condition is not considered premalignant, it can involve the bladder extensively and is difficult to eradicate.
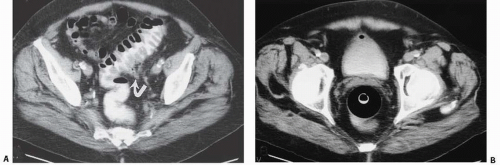
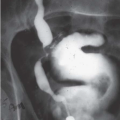
Diverticula
Bladder diverticula may occur as a result of bladder outlet obstruction, or rarely, a congenital deficiency in bladder musculature adjacent to the ureterovesical junction (Fig. 15.19). The latter is termed a Hutch diverticulum and is commonly associated with ipsilateral vesicoureteral reflux. Bladder diverticula are not infrequently encountered in adults, usually being acquired and resulting from functional or mechanical bladder outlet obstructions (the former due to neurogenic bladders and the latter often due to prostatic enlargement). Bladder diverticula due to outlet obstruction are rare in children but may occur in the clinical setting of urethral valves. Neurogenic dysfunction of the bladder is another cause of multiple diverticula in children.
Multiple bladder diverticula are common, usually arising from the lateral walls and rarely arising near the bladder dome. A widenecked diverticulum empties readily, but a narrow-necked diverticulum empties slowly and is more likely to have residual urine. A large bladder diverticulum may displace the bladder to the opposite side and may be larger than the bladder. In such cases, the bladder is identified on CT by its thickened wall, whereas the diverticulum has a smooth thin wall.
There may be a higher incidence of tumor in bladder diverticula than in the bladder lumen itself, explained by stasis of carcinogen-laden urine in the diverticulum. There is a tendency for such tumors to spread outside the bladder more rapidly than bladder lumen tumors because the walls of false diverticula consist only of urothelium protruding between muscle bundles and are not surrounded by detrusor muscle.
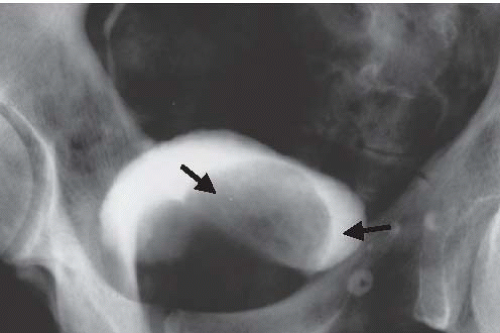
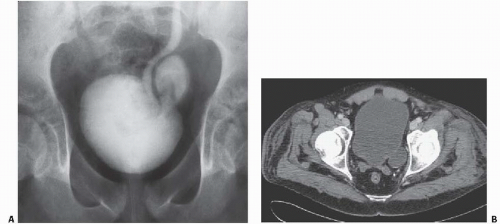 FIGURE 15.19. Hutch diverticulum. A cystogram demonstrates reflux into the left ureter and a diverticulum adjacent to the ureter. |
Ultrasound is effective in the assessment of bladder diverticula. Echo-free outpouchings from the bladder are readily seen (Fig. 15.20), and filling defects within a diverticulum such as stones or tumors also are visible. Tumors within a diverticulum may present as soft-tissue masses in the diverticulum or only as thickening of the diverticulum wall, whereas stones may produce typical shadowing. The tumor may on occasion obstruct the mouth of the diverticulum. In such cases, the diverticulum will not be opacified on cystography or other contrast-enhanced studies (Fig. 15.21).
CT is an excellent method of assessing bladder diverticula (Fig. 15.22). Stones are easily seen on unenhanced CT images as highattenuation filling defects, whereas a tumor presents as a soft-tissue mass arising from the diverticulum wall and protruding into the contrast medium-filled diverticulum (Fig. 15.23). MRI is also useful in evaluating tumors arising in bladder diverticula (Fig. 15.24).
Herniation
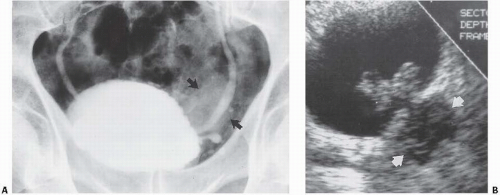
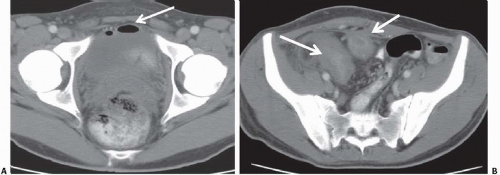
Patients with herniation of the bladder into or through the inguinal canal present with swelling in the groin or scrotum that increases as the urinary bladder fills and subsides as the patient voids. Bladder herniation most commonly is paraperitoneal in location, with the bladder remaining extraperitoneal and medial to a true inguinal hernia sac. Herniation of the bladder also can be within a true hernia sac or can occur totally extraperitoneally with the peritoneum remaining in the abdomen. The bladder also may herniate through the femoral canal into the thigh or through various incisional hernias.
Bladder herniation can occur in varying degrees and can usually be easily seen when the bladder is filled with contrast medium during cystography (Fig. 15.25) although an upright film is occasionally necessary to make the diagnosis. Bladder herniation may occasionally be seen as an incidental finding on CT and is usually easy to detect when the bladder lumen contains contrast material (Fig. 15.26). However, in some cases this condition may be quite subtle (Fig. 15.27).
Cystocele and Stress Incontinence
A cystocele is defined as prolapse of the bladder into the anterior portion of the vagina and is seen radiographically as descent of the bladder below the symphysis pubis. A cystocele results from laxity of the pelvic floor muscles and is usually only one component of pelvic floor dysfunction, which may include a cystocele, hypermobile bladder neck, vaginal vault prolapse, rectocele, enterocele, sigmoidocele, or rectoanal intussusception. Conditions that predispose to pelvic floor dysfunction include multiparity, obesity, prior pelvic surgery, excessive straining, connective tissue disorders, neuropathy, and advancing age. MRI is useful for evaluating the pelvic floor without the need for bladder or rectal opacification.
Fistulae
Enterovesical and Colovesical Fistulae
Fistulae to the bowel may present with fecaluria, pneumaturia, or persistent urinary tract infection, resulting from a microscopic communication between the bladder and bowel that allows passage of bacteria, but not gas or stool.
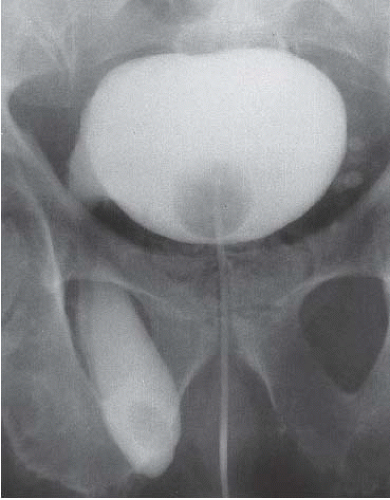 FIGURE 15.25. Bladder herniation. Cystogram shows part of the bladder to have herniated into the scrotum. |
When small bowel is involved, the term enterovesical fistula is preferred. Such fistulae are usually caused by Crohn disease. The rectosigmoid colon is the most frequently involved segment of the large bowel and communications with the bladder are called colovesical fistulae. The most common cause of colovesical fistulae in the United States is diverticulitis. Colon cancer is the second most common cause. Other causes of fistulae between the bladder and the small or large bowel include penetrating trauma, surgical misadventures, other inflammatory processes such as appendiceal abscess or pelvic inflammatory disease, and bladder infections with granulomatous conditions such as schistosomiasis and tuberculosis.
The presence of gas in the bladder lumen may indicate a fistula. However, recent instrumentation by catheterization or cystoscopy, or acute cystitis with E. coli, may also produce such a finding. Gas in the bladder wall is found only in emphysematous cystitis.
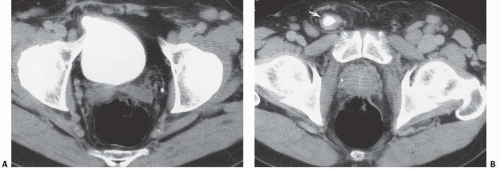 FIGURE 15.26. Bladder herniation. A, B: Bladder herniating into the inguinal canal (arrow) was found as an incidental finding on this contrast-enhanced CT examination. |
Cystography can demonstrate the actual fistula between bladder and bowel in approximately 50% of cases. Bowel contrast studies are positive in even fewer cases. However, when the fistulous tract is widely patent, imaging of the contrast-filled bladder or bowel demonstrates the communication. Cystoscopy may show the actual fistula in some instances; however, the diagnosis may be difficult. The most typical cystoscopic finding is an isolated area of inflammation of the bladder wall.
CT is the most sensitive imaging study for detection of a bladder fistula, with the most commonly encountered finding being the presence of a small amount of air in the bladder lumen. This finding is seen in most patients (Fig. 15.28) and an equal number of patients exhibit focal bladder wall thickening, associated with focal thickening of a loop of small or large bowel adjacent to the bladder (Fig. 15.29). The diagnosis can usually be inferred from these findings in patients with predisposing conditions. As with cystography and barium enema, CT shows flow of contrast medium through the fistula in no more than 50% of cases.
Vesicovaginal Fistula
Vesicovaginal fistulae result in painless constant dribbling of urine from the vagina. They are a known complication in patients undergoing hysterectomy or other pelvic surgery, especially when preoperative radiation was used. Seventy-five percent of cases occur after hysterectomy for a benign condition, whereas the remainder are found after hysterectomy in patients with a pelvic malignancy. Vesicovaginal fistulae, because of their typically large size, are usually relatively easy to demonstrate on imaging studies. To visualize the contrast-filled vagina during cystography, oblique, lateral (Fig. 15.30), or postvoiding films may be necessary. Vesicovaginal fistulae may also be detected with CT and MRI (Fig. 15.31)
COMMON CAUSES OF BLADDER FISTULAE
Enterovesical
Crohn disease
Colovesical
Diverticulitis
Colon carcinoma
Vesicovaginal
Cervical cancer treated with radiation
Posthysterectomy
Deviations and Impressions
Various conditions in the pelvis extrinsic to the bladder can cause an impression on the bladder wall, can circumferentially compress the bladder, or can deviate the bladder to one side.
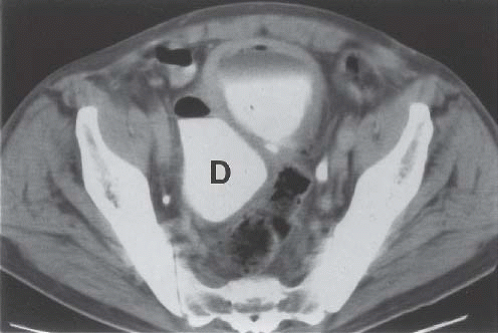
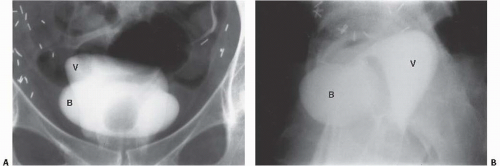
Displacement or compression of the bladder implies a pelvic mass. Common lesions that may indent the bladder include enlarged pelvic lymph nodes, tumors arising from the colon, reproductive organs, or mesenchymal tissues, presacral teratomas, iliac artery aneurysms, bladder diverticula, hematomas (Fig. 15.32), and abscesses. An anterior meningomyelocele behind the bladder can deviate the bladder anteriorly. These masses are usually accompanied by pathognomonic changes in the sacrum, including a wellcorticated concavity on one side of the sacrum and deviation of the sacrum to the contralateral side (scimitar sacrum). Other cystic lesions that can indent the bladder from behind include seminal vesicle and müllerian duct cysts.
Circumferential compression of the bladder results in a configuration commonly known as a “teardrop” or “pear-shaped” bladder. Processes that can surround the bladder include the normal variant of a narrow pelvis with prominent iliopsoas muscles such as can be found in young male patients. Diffuse pelvic hematoma, another cause of teardrop bladder, is nearly always seen in the setting of recent surgery or trauma (Fig. 15.33), although spontaneous bleeding can occur in patients with bleeding diatheses or on anticoagulation therapy.
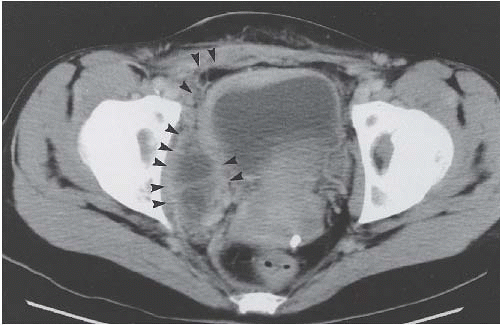
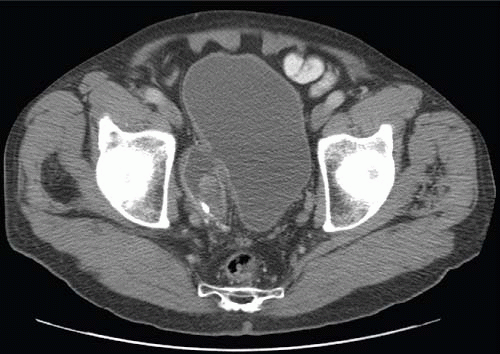
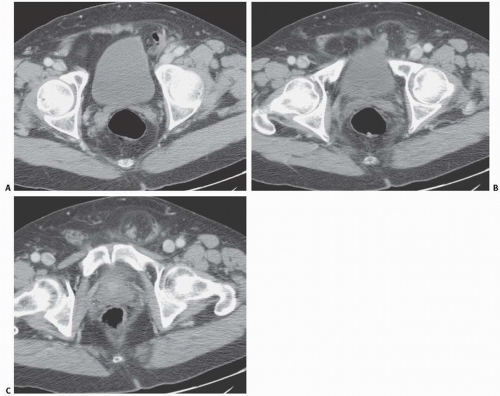
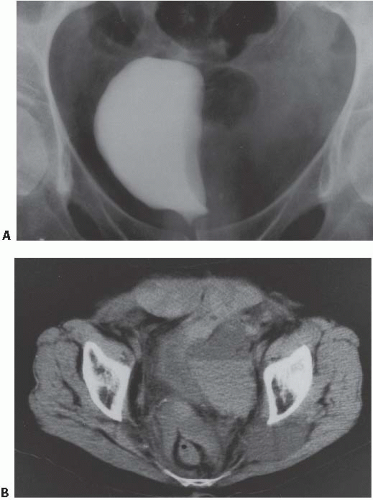
Another condition resulting in bladder compression is pelvic lipomatosis. This results from a proliferation of mature, unencapsulated fat within the pelvis. No etiology for this condition has been identified. Radiographically, plain films may show a lucency in the pelvis corresponding to the increased fat. The distal ureters are medially deviated, but the mid ureters may be laterally displaced due to the elevation of the lower urinary tract. Additionally, the ureters may be obstructed in severe cases. On barium enema, the rectosigmoid colon is straightened and narrowed. The excess pelvic fat responsible for compressing the colon and bladder can be easily confirmed with CT (Fig. 15.34).
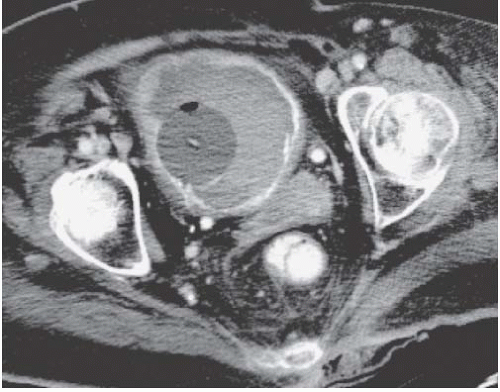
Bilateral pelvic lymph node enlargement due to metastatic disease may also result in a teardrop or vertical configuration of the bladder (Fig. 15.35). This is usually found in patients with lymphoma. Other tumors that drain to the pelvic lymph nodes, such as prostate cancers, do not typically produce bulky lymph node metastases. Other causes of teardrop bladder include development of bilateral external iliac artery aneurysms, massive venous collaterals in the pelvis in patients with inferior vena cava obstruction, retroperitoneal fibrosis occurring deep in the pelvis, and lymphoceles that form after pelvic lymph node dissection.
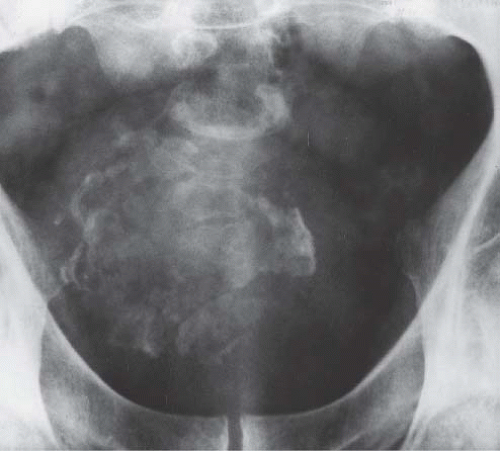
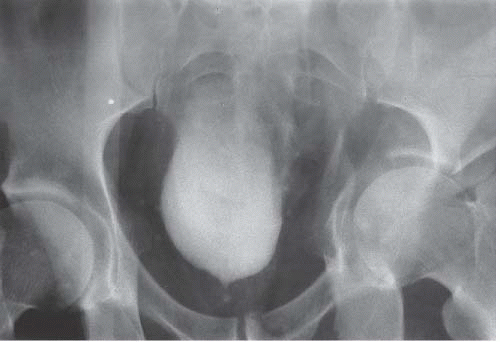 FIGURE 15.33. Pelvic fracture. Hematomas from pelvic fracture compress the bladder and create a teardrop configuration of the bladder on this anteroposterior image obtained during a cystogram.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|