This article reviews the current status and trends in the treatment of primary gliomas, focusing predominantly on glioblastoma. It also discusses the current standard of care and new targeted agents currently under investigation. Furthermore, the concepts of pseudoprogression and pseudoresponse are introduced, which are new imaging correlates that have significant impact on understanding of the disease.
Of the approximately 23,000 new cases of malignant central nervous system neoplasms diagnosed annually in the United States, more than half will be categorized as glioblastoma, which is the most aggressive subtype of the malignant gliomas. Despite the application of radiation, chemotherapy, and modern aggressive surgical approaches, average survival is approximately 1 year.
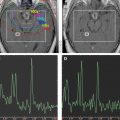
However, several strategies are under investigation to improve outcome. The first involves attempts to enhance the benefit from radiation, which is the most powerful nonsurgical tool. Attempts at dose escalation more than 6000 cGy have resulted in increased toxicity without a survival benefit. Therefore, radiation sensitizers have been explored. To improve local control and limit toxicity to normal brain tissue, novel imaging techniques (eg, chemical shift imaging) are being explored to better define radiotherapy fields.
Other confounds to therapy relate to drug resistance or interactions (eg, P450 induction or inhibition) and overcoming the blood–brain barrier with systemic drug therapies. The alkylating agent temozolomide has emerged as a key antineoplastic agent that may also have radiosensitizing effects. Ongoing clinical trials are evaluating temozolomide in combination with other systemic agents (eg, motexafin gadolinium, mammalian target of rapamycin [mTOR] inhibitors, farnesyl-transferase inhibitors, tyrosine kinase inhibitors [TKIs], and antiangiogenesis agents), which have shown promising activity in combination with radiotherapy.
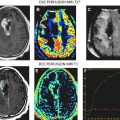
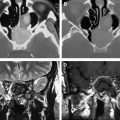
Additionally, the ability to assess progression radiographically has become an ever-increasing and daunting task. It has become increasingly apparent that early and late radiotherapy effects can be mistaken for progressive disease with MRI and MR spectroscopy, often called pseudoprogression . Pseudoprogression is especially seen with combined modality approaches (eg, temozolomide, radiotherapy), occurring in up to 20% of patients.
Moreover, the potential for a pseudoresponse in some clinical scenarios is now recognized. The use of vascular endothelial growth factor (VEGF) inhibitors, which affect vascular permeability, can reduce contrast enhancement on MRI and CT scans as a result of diminished penetration of contrast agents. Therefore, scan changes during treatment with VEGF inhibitors may overestimate the extent of response when using traditional imaging criteria. These considerations complicate the care management of individual patients, interpretation of clinical trial data, and development strategies for future clinical investigation.
This article identifies the current status and trends for the treatment of gliomas (focusing on glioblastoma), and describes some of the difficulties and controversies.
Newly diagnosed glioblastoma: current standard of care
Until recently, the median overall survival of patients who had glioblastoma generally ranged from 10 to 12 months, with fewer than 10% of patients surviving 2 years. Meta-analyses showed a modest benefit to the use of adjuvant nitrosourea-based chemotherapy, and early trials showed that nitrosoureas increased the likelihood of surviving more than 18 months. Strategies for newly diagnosed glioblastoma have varied. Gilbert and colleagues used preradiation temozolomide as an adjunct to treatment. Although preradiation temozolomide resulted in an impressive 41% response rate, response duration was short, and median overall survival was in the typical range of 1 year.
In 2003, the use of carmustine (BCNU)-impregnated wafers (Gliadel) in a phase III placebo-controlled study of patients who had World Health Organization (WHO) grade III and IV gliomas suggested a survival advantage ; however, when patients who did not have glioblastomas were removed from the analysis, the study was underpowered to show a statistically significant result. Furthermore, no significant improvement in progression-free survival was seen, which many neurooncologists consider a surrogate marker for overall survival. In addition, wafer implantation may lead to increased difficulty with wound healing and infection, and confound MRI interpretation. Nonetheless, Gliadel wafers are an approved standard of care for newly diagnosed glioblastoma.
A significant therapeutic breakthrough occurred when Stupp and co-workers reported results of the use of daily temozolomide (75 mg/m 2 ) during radiotherapy (approximately 6 weeks), followed by 6 months of adjuvant therapy at 150 to 200 mg/m 2 on days 1 through 5 for a 28-day cycle. Median survival in this phase II study was 16 months. The rationale for this approach included the possibility of radiosensitization, for which preclinical support now exists.
The concept of daily dosing was also supported by observations showing that extended daily administration of temozolomide depletes the DNA-repair enzyme O-6-methylguanine-DNA methyltransferase (MGMT). MGMT is a proposed resistance mechanism for temozolomide. After MGMT removes a methyl group from DNA (deposited by temozolomide), it is no longer active; hence, MGMT is called a suicide enzyme .
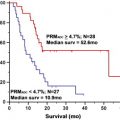
This concept was tested in the recurrent setting with encouraging results preliminarily. Use of adjuvant temozolomide starting 1 month postradiotherapy was predicated on the inherent antitumor activity of the drug. This study showed a promising 2-year survival rate of 31% and led to a confirmatory (European/Canadian) phase III trial comparing the phase II regimen with radiotherapy alone. This randomized phase III trial confirmed the earlier phase II study, showing that the experimental regimen (now colloquially known as the Stupp Regimen ) produces a statistically significant increase in median overall survival of 12.1 versus 14.6 months and, more impressively, an improved 2-year survival of 10% versus 26%. Longer follow-up also shows that the benefit was sustained after 4 and 5 years.
A retrospective analysis of tissue samples from this study also suggested that MGMT promoter methylation correlates with improved outcome. When the promoter region of the MGMT gene is methylated, the gene is silenced (ie, transcriptionally inactive and therefore ultimately not producing MGMT enzyme). Patients who had tumors harboring MGMT promoter methylation who were randomized to receive temozolomide had a median survival of 15.3 months and a 2-year survival rate of 46%, compared with 12.7 months and 13.8% for patients who had tumors not exhibiting promoter methylation.
However, several observations suggest the biology may be more complicated. First, patients in the control arm (who received radiotherapy alone at diagnosis and of whom approximately 60% received temozolomide at recurrence), promoter methylation also resulted in clinical benefit (ie, overall survival of 15.3 versus. 11.8 months for patients who had an unmethylated promoter). Other studies have also shown that MGMT promoter methylation correlates with a survival benefit for patients treated with radiotherapy alone.
In addition, immunohistochemistry for MGMT expression does not correlate with survival. Therefore, it is possible that MGMT promoter methylation contributes to prognosis through MGMT inactivation, but may also serve as a marker of as unidentified prognostic factors. Other markers under investigation for prognostic value include epidermal growth factor receptor (EGFR) amplification, tenascin expression, phosphatase and tensin homolog on chromosome 10 (PTEN), loss of chromosome 10, mutation or loss of the P53 gene, and expression of YKL-40. In addition, the finding of MGMT promoter methylation status as a putative marker of response to therapy and outcome has stimulated further laboratory and clinical evaluation of other temozolomide resistance mechanisms, such as the base excision repair pathway. This pathway depends on a group of enzymes in the poly (ADP-ribose) polymerase (PARP) family that provide the energy needed for base excision. PARP-1 inhibitors have been tested preclinically and have moved into phase I and II testing.
Targeted agents in recurrent disease
The marked molecular heterogeneity of glioblastomas provides another opportunity for directed therapy with novel targeted agents based on the recent elucidation of signaling pathways. As single agents, these drugs have shown limited efficacy ( Table 1 ).
| Agent | Class | N | Response Rate | Median OS | Median PFS/TTP | 6-Month PFS Rate |
|---|---|---|---|---|---|---|
| TMZ (naïve) | Alkylator | 382 | 5%–16% | 5–8 mo | 2–3 mo | 18%–21% |
| BCNU | Alkylator | 40 | 15% | 7.5 mo | 3.1 mo | 17.5% |
| Gefitinib | EGFR | 92 | 0% | 10 mo | 2 mo | 9%–13% |
| Erlotinib | EGFR | 78 | 0%–8% | 10 mo | 2–3 mo | 0%–17% |
| Imatinib | PDGFR | 83 | 6% | 5.9 mo | 1.8 mo | 3%–16% |
| CCI-779 | mTOR | 106 | <5% | 4.4 mo | 2.3 mo | 2%–8% |
| Pooled phase II | Various | 225 | 6% | 5.7 mo | 2 mo | 15% |
| Pooled phase II (no TMZ) | Various, 1998–2002 | 291 | <7% | 6.0 mo | 1.6 mo | 9% |
However, responses do occur, suggesting that patient subpopulations could be identified that may uniquely benefit from a given drug. For example, the status of the PTEN gene, lost or otherwise inactivated in most glioblastomas, is a key regulator of the Akt signal transduction pathway. PTEN loss or inactivation leads to Akt activation, which is involved in resistance to apoptosis and acceleration of cell proliferation, and may have implications for prognosis and response to EGFR TKIs.
In a retrospective study, Mellinghoff and colleagues observed that a constitutively activated form of EGFR (ie, the mutant EGFR variant III [ EGFRvIII ]), in association with retained PTEN expression, predicted response to the EGFR TKIs erlotinib and gefitinib. Although some have questioned this result, Haas-Kogan and colleagues also reported that EGFR amplification and low levels of AKT activity (consistent with PTEN retention) predicted response. Prospective trials to accrue patients who have EGFR mutant disease are planned.
Platelet-derived growth factor (PDGF) overexpression has been described in up to 66% of glioblastomas. However, clinical trials performed without regard to molecular prescreening have shown negative results. Taken collectively, these observations provide the basis for prospective trials involving patients selected based on the molecular characterization of their tumors.
Because results of trials using drugs with one target have been disappointing, current efforts involve combining a single molecularly targeted agent with cytotoxic chemotherapy or radiotherapy. Furthermore, the combination of two targeted agents is also an attractive concept (eg, a receptor tyrosine kinase [eg, EGFR] inhibitor combined with a signal transduction [eg, mTOR] modulator). Another strategy involves the use of single agents with multiple targets. All of these approaches are being pursued, although some results have been disappointing.
One of the most promising targets in recent studies has been the vascular endothelial growth factor receptor (VEGFR), which is overexpressed in glioblastomas. Bevacizumab is a monoclonal anti-VEGF antibody that has been tested either alone or in combination with chemotherapy or radiotherapy in several phase II trials for patients who had recurrent glioblastomas ( Table 2 ).
| Agent(s) | Study Type | N | Response Rate | Median OS | Median PFS | 6-month PFS Rate | Notable Major Toxicity |
|---|---|---|---|---|---|---|---|
| Bevacizumab + irinotecan | Phase II | 35 | 56 | 9.7 mo | 5.5 mo | 46% | 1 CNS bleed, 4 PE/DVT, 4 severe fatigue |
| Bevacizumab + irinotecan/other | Retrospective | 23 | 34% | 8.2 mo | 5.5 mo | 42% | 2 CNS bleeds (asymptomatic), 4 PE, 1 SMV thrombosis 1 colon perforation |
| Bevacizumab | Phase II | 85 | 28% | 9.2 mo | Not described | 43% | 2.4% fatal AE |
| Bevacizumab | Phase II | 48 | 35% | 7.3 mo | 3.7 mo | 29% | Thromboembolism (12.5%), hypertension (12.5%), hypophosphatemia (6%), thrombocytopenia (6%), bowel perforation (2%); 0 CNS bleeds |
| Bevacizumab + irinotecan | Phase II | 82 | 38% | 8.7 mo | Not described | 50% | 1.3% fatal AE |
| Bevacizumab + re-radiation | Pilot | 20 GBM | 50% | 12.5 mo | 7.3 mo | 65% | 1 each: CNS bleed, bowel perforation, gastrointestinal bleed, wound dehiscence; no radionecrosis |
| Cediranib (AZD2171) | Phase II | 31 | 56% | 7.3 mo | 3.8 mo | 26% | Hypertension, headache |
| Aflibercept | Phase II | 32 | 30% | Not described | Not described | Not described | 1 CNS ischemia, 1 systemic hemorrhage, others |
Drawing from the experience in colon cancer, in which irinotecan (CPT11) has activity, bevacizumab has been combined with CPT11 for recurring glioblastoma, showing responses in up to 56% of patients. However, a portion of the biologic rationale for CPT-11/bevacizumab in colon cancer (ie, increased drug penetration and disruption of early blood vessels) may not be entirely applicable to glioblastoma. Whether bevacizumab plus CPT11 has superior efficacy to bevacizumab alone for glioblastoma remains unknown.
Several studies suggest that bevacizumab monotherapy is a reasonable therapeutic approach. A phase II study in a pediatric population showed minimal bevacizumab activity, suggesting the possibility of age-related biology. Other inhibitors of VEGF/VEGFR are also in active trials, including aflibercept (VEGF-Trap), pazopanib, and cedirinib (AZD2171).
Targeted agents in recurrent disease
The marked molecular heterogeneity of glioblastomas provides another opportunity for directed therapy with novel targeted agents based on the recent elucidation of signaling pathways. As single agents, these drugs have shown limited efficacy ( Table 1 ).
| Agent | Class | N | Response Rate | Median OS | Median PFS/TTP | 6-Month PFS Rate |
|---|---|---|---|---|---|---|
| TMZ (naïve) | Alkylator | 382 | 5%–16% | 5–8 mo | 2–3 mo | 18%–21% |
| BCNU | Alkylator | 40 | 15% | 7.5 mo | 3.1 mo | 17.5% |
| Gefitinib | EGFR | 92 | 0% | 10 mo | 2 mo | 9%–13% |
| Erlotinib | EGFR | 78 | 0%–8% | 10 mo | 2–3 mo | 0%–17% |
| Imatinib | PDGFR | 83 | 6% | 5.9 mo | 1.8 mo | 3%–16% |
| CCI-779 | mTOR | 106 | <5% | 4.4 mo | 2.3 mo | 2%–8% |
| Pooled phase II | Various | 225 | 6% | 5.7 mo | 2 mo | 15% |
| Pooled phase II (no TMZ) | Various, 1998–2002 | 291 | <7% | 6.0 mo | 1.6 mo | 9% |
However, responses do occur, suggesting that patient subpopulations could be identified that may uniquely benefit from a given drug. For example, the status of the PTEN gene, lost or otherwise inactivated in most glioblastomas, is a key regulator of the Akt signal transduction pathway. PTEN loss or inactivation leads to Akt activation, which is involved in resistance to apoptosis and acceleration of cell proliferation, and may have implications for prognosis and response to EGFR TKIs.
In a retrospective study, Mellinghoff and colleagues observed that a constitutively activated form of EGFR (ie, the mutant EGFR variant III [ EGFRvIII ]), in association with retained PTEN expression, predicted response to the EGFR TKIs erlotinib and gefitinib. Although some have questioned this result, Haas-Kogan and colleagues also reported that EGFR amplification and low levels of AKT activity (consistent with PTEN retention) predicted response. Prospective trials to accrue patients who have EGFR mutant disease are planned.
Platelet-derived growth factor (PDGF) overexpression has been described in up to 66% of glioblastomas. However, clinical trials performed without regard to molecular prescreening have shown negative results. Taken collectively, these observations provide the basis for prospective trials involving patients selected based on the molecular characterization of their tumors.
Because results of trials using drugs with one target have been disappointing, current efforts involve combining a single molecularly targeted agent with cytotoxic chemotherapy or radiotherapy. Furthermore, the combination of two targeted agents is also an attractive concept (eg, a receptor tyrosine kinase [eg, EGFR] inhibitor combined with a signal transduction [eg, mTOR] modulator). Another strategy involves the use of single agents with multiple targets. All of these approaches are being pursued, although some results have been disappointing.
One of the most promising targets in recent studies has been the vascular endothelial growth factor receptor (VEGFR), which is overexpressed in glioblastomas. Bevacizumab is a monoclonal anti-VEGF antibody that has been tested either alone or in combination with chemotherapy or radiotherapy in several phase II trials for patients who had recurrent glioblastomas ( Table 2 ).