and Ashutosh Dash2
(1)
Nuclear Security and Isotope Division, Oak Ridge National Laboratory, OAK RIDGE, USA
(2)
Isotope Production and Applications Division, Bhabha Atomic Research Centre, Mumbai, India
11.1 Introduction
HCC represents the sixth most common malignancy worldwide and the third most common cause of cancer-related mortality causes estimated to result in half a million deaths annually (Parkin et al. 2005; Bosch et al. 2004; El-Serag et al. 1992). The most relevant risk factors for HCC include chronic hepatitis C (HCV) infection, hepatitis B (HBV) infection, alcoholic cirrhosis, and nonalcoholic fatty liver (steatohepatitis) (Deuffic et al. 1998). While local therapy by surgical resection with tumor-free margins (llovet et al. 2003) or liver transplantation (Bismuth and Majno 2000) represent potentially curative treatments, only one third of patients with HCC are suitable candidates for hepatic resection, due to either anatomic location of the lesion(s), lesion size, the number of lesions, concurrent nonmalignant liver disease, or insufficient hepatic reserve. Although another therapeutic option widely practiced for primary and secondary hepatic malignancies is systemic chemotherapy, it is confined to tumor response rates of less than 30 % (Vassiliou et al. 2007; Dawson et al. 2000). The use of external beam radiotherapy making use of intensity-modulated radiotherapy (IMRT) for the treatment of primary of metastatic liver tumors is often of limited use in patients with diffuse, multiple lesions, due to the low tolerance of normal liver parenchyma to radiation. The cytotoxic dose required to eradicate solid tumor is estimated to be ~70 Gy, which is far greater than the liver tolerance dose of 35 Gy that could be delivered to the whole liver by IMRT in 1.8 Gy/day fractions (Dawson et al. 2000; Kennedy et al. 2007). Therefore, palliative management has emerged as the mainstay of therapy for most patients with inoperable HCC (Cammà et al. 2002; Cabibbo et al. 2010). Given the limited efficacy of external beam radiotherapy approaches for the treatment of HCC, a number of techniques to deliver targeted tumor radiation by means of radiopharmaceuticals have thus been developed and evaluated.
Transarterial chemoembolization (TACE) is a locoregional therapy that involves the injection of chemotherapeutic agents, with or without lipiodol and embolic agents, into the branch of the hepatic artery that feeds the tumor. This regional therapy is widely used for unresectable HCC to achieve a higher local concentration of the agents with limited systemic toxicity (Geschwind 2002). In TACE of HCC, single-agent doxorubicin is most commonly used in Europe and Japan, while the combination of mitomycin C, doxorubicin, and cisplatin is more popular in the USA. Single or combination agents are typically emulsified in lipiodol, an oily contrast agent believed to increase intratumoral retention of the cytotoxic agent (Kennedy and Sangro 2014).
Radionuclide therapy for HCC thus appears to be an effective therapeutic option, for the treatment of large inoperable HCC, particularly with portal vein thrombosis and of small inoperable tumors unsuitable for any reason for percutaneous therapy embolization, with or without chemotherapeutic agents. This technology is also practiced as neoadjuvant therapy before hepatic transplantation to reduce the risk of recurrence in the graft or before hepatic resection to shrink the tumor size or as an adjuvant therapy after surgery or percutaneous ablative therapy to reduce the risk of recurrence. The selection of an appropriate treatment option for HCC is very patient specific/personalized and depends on careful assessment of tumor staging and assessment of the underlying liver disease. Strategies for delivery of radionuclide to the liver for the purpose and targeting the tumor can be accomplished by three distinct modalities. Direct intratumor implantation of the radionuclide is possible and parenteral injection of radiolabeled antibodies specific to HCC antigens (radioimmunotherapy) is also an option. The most appealing option for HCC treatment is trans-arterial radioisotope therapy (TART) involving direct administration of radionuclide through the hepatic artery directly into the tumor. The major blood supply to hepatocytes in the liver parenchyma is via the portal vein, and tumors are generally fed via the hepatic arterial supply (Fig. 11.1) which represents an accessible avenue to administer these radioactive agents.
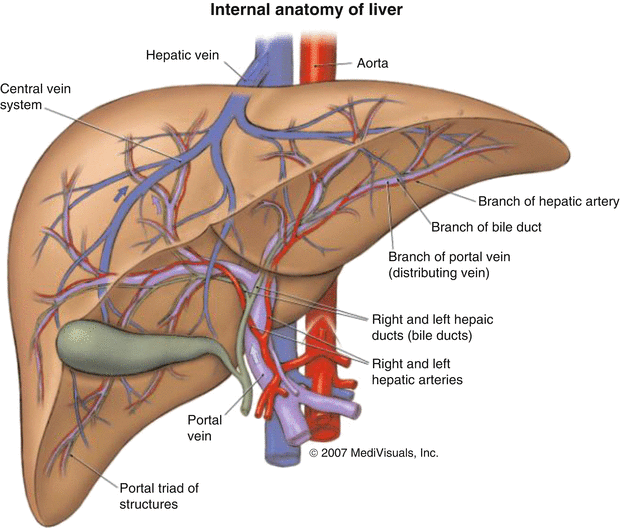

Fig. 11.1
Illustration of the hepatic blood supply
11.2 Direct Intratumor Implantation
Direct introduction of the radionuclide into the target lesion tumor under ultrasonographic guidance is one of the procedures often used with varying success (Tian et al. 1996; Goh et al. 2007; Kim et al. 2006). Major drawbacks of this modality is the growing concern regarding the leakage of radioactivity outside the tumor into the peritoneal cavity, difficulties in achieving a homogenous distribution of radioactivity over a large tumor mass, and difficulties in treating percutaneously inaccessible tumors.
11.3 Radioimmunotherapy
Radioimmunotherapy (RAIT) is another therapeutic approach for patients with HCC and a variety of antibodies such as anti-CEA antibodies and anti-AFP, and anti-ferritin labeled with 131I or 90Y have been widely evaluated for this purpose (Order et al. 1985, 1986, 1991; Fan et al. 1992; Liu et al. 1989; Tang et al. 1990; Abrams et al. 1998). Ferritin is both a normal tissue- and tumor-associated protein which acts as an immunosuppressant and as an iron storage protein. As a tumor-associated protein, it is related to virally induced tumors. As one example, the State Food and Drug Administration in the People’s Republic of China (PRC) has approved 131I-metuximab (Licartin, Chengdu Huasun Bio-Tech) as a therapeutic anti-HCC radioimmunological agent for the treatment of unresectable HCC. As another example, 131I-metuximab is an iodine 131I-labeled murine monoclonal antibody (mAb) HAb18 F(ab’)2 fragment against the HCC-associated antigen HAb18G/CD147. The mAb HAb18 (immunoglobulin G1) has been obtained by use of a cell suspension extracted from fresh human HCC tissues to immunize BALB/c mice and for hybridoma preparation. Its HAb18G/CD147 antigen was highly expressed on HCC cells and is a member of the CD147 family (Chen et al. 2006; Zhang et al. 2006; Zeng et al. 2002). Although some positive results have been reported, this form of treatment has several limitations which include the heterogeneity of tumor cells and not all the cells express the same antigen. In addition, there is poor localization of murine immunoglobulin in tumor tissue, probably resulting from the large variation of tumor vascularity and irregular penetration of antibodies into large tumors which contain a necrotic center. Bone marrow toxicity caused by the presence of radiolabeled antibodies in the peripheral circulation can also be encountered, and host reaction to the murine antibodies may lead to the potentially dangerous development of human anti-mouse antibodies (HAMA).
11.4 Trans-arterial Radioisotope Therapy (TART)
In contrast, trans-arterial radioisotope therapy is a promising technique that exploits the unique hepatic dual blood supply, involving both the portal vein and hepatic artery. In a normal functioning liver, the portal vein is responsible for supplying the majority of blood to the liver parenchyma (75–80 %), with the hepatic artery providing only 20–25 %. This balance is significantly altered in HCC where the hepatic artery solely provides perfusion to the tumor and liver parenchyma derives >75 % of its blood supply from the portal vein. HCCs are rich in vasculature and almost exclusively dependent on arterial blood supply. This anatomical configuration precisely provides the basis for the development of trans-arterial radioisotope therapy for the treatment of HCC. Radiolabeled microspheres with a diameter between 20 and 50 μm are selectively injected into the hepatic vessels supply tumors and selectively congregate in the hepatic end-arterioles, allowing localized delivery of therapeutic doses, while sparing the surrounding liver parenchyma (Ackerman et al. 1970). The goal of TART is to cause tumor necrosis and tumor control while preserving as much functional liver tissue as possible and so to prolong life. Thus, it is essentially a flow-directed mode of radionuclide treatment that is dependent on neoangiogenesis.
The advantages of TART include radioisotopic tumor-destroying effects of the radioisotope which are independent on the HCC cellular characteristics since it is not necessary for the radioisotope to actually be taken up by the tumor cells for the desired destructive irradiation of the adjacent tumor cells. Since the hepatic artery is usually not embolized during this procedure, it can be safely used in patients with compromised liver function or portal vein thrombosis, which are usually observed in a large number of patients with locally advanced disease. The use of β-emitting therapeutic radioisotopes which also emit gamma photons, such as 131I, 188Re, 166Ho, etc., makes external dosimetry possible which can help in individualizing patient treatment and thus help avoid or reduce side effects/toxicity and help achieve better tumor response by administering the tumoricidal dose (in cases of radioisotopes having gamma emissions). Also, prophylactic irradiation of apparently normal liver parenchyma can reduce the risk of recurrence (adjuvant/neoadjuvant role).
11.5 Selection of Radionuclide for TART
Therapeutic radioisotopes that can be used for the treatment of HCC should meet several criteria which include the emission of radioactive particles, and β−-emitting radionuclides have been of choice for this application because of their regional energy deposition and the wide selection of reactor-produced candidates (Chap. 5). The energy of beta radiation should be adequately intense to create a zone of high radiation exposure confined to the vicinity of the tumor while minimizing non-tumorous hepatic parenchymal exposure to tolerable levels. For these reasons, the use of high-energy β− radiation with low mean free path is desirable. The physical half-life of the radionuclide is also an important issue and should be relatively short but effective in delivering a cytotoxic radiation dose to the tumor. It also is desirable to use radionuclides with high specific activity, but this is not a necessity and depends on the mass load of the particles. Higher-specific-activity radionuclides provide flexibility to bound desired amount of activity to the embolic particles. The chemical characteristic of the radionuclide should also permit efficient incorporation into a wide range of embolic particles. Finally, the detectable percentage of gamma emission for imaging is advantageous to assess targeting, residence time, and dosimetry to relate dose delivery with response.
11.6 Selection of Microspheres
Radiolabeled microspheres for intra-arterial therapy should (Harbert 1996) be mechanically strong enough to withstand the capillary force during their passage through the capillary network. Excellent chemical stability is also required to circumvent elution of the radiolabel, macrophage removal, or radiolysis to prevent bone marrow toxicity. Uniform particle size is necessary and the density of the particles should be greater than or equal to 1 to prevent settling or streaming. The procedure should be a simple and efficient method for radiolabeling, and the particles should of course be nontoxic, biocompatible, and preferably biodegradable.
11.7 Common Microsphere Materials
Given the scope of using TART for the treatment of HCC, several materials for fabrication of microspheres with a diameter between 20 and 50 μm have been developed and evaluated. Both glass, resins, albumin, and polymers are examples of materials which have been used. Selection of glass is primarily based on its resistant to radiation damage. It is also highly insoluble, nontoxic and spheres of uniform sizes can be readily prepared (Leung et al. 1994). It is possible to produce glass microspheres with negligible leaching (Ehrhardt and Day 1987). While these aspects are appealing, their high density and their non-biodegradability have emerged as issues which have to be further evaluated (Ho et al. 1997; Mumper et al. 1991). The relatively high-density glass is one of the major barriers that increase the likelihood of intravascular settling (Turner et al. 1994). Resin-based microspheres are attractive for radioembolization as they are insoluble and nontoxic, with commercial availability of uniform sizes, and radionuclides are easily incorporated with high labeling yield. A broad range of commercially available resins such as Bio-Rex 70, Cellex-P, Chelex 100, Sephadex SP, and AG 50W-X8 have been used (Ho et al. 1998; Schubiger et al. 1991; Wang et al. 1998; Burton et al. 1998). Another advantage is that the resin particles do not undergo degradation in vivo. Human serum albumin (HSA) has been widely used as these particles are biocompatible and biodegradable. Both 188Re and 90Y have been bound to HSA by suspending in sodium acetate buffer and incubated at room temperature (Lau et al. 1994). One of the major impediments of using HSA is the time-consuming labeling process and generally poor radiolabeling yields. Major advantages of polymer-based microspheres are their near-plasma density, biodegradability, and biocompatibility. The poly (l-lactic acid) (PLLA) microspheres have been extensively used for radioembolization of holmium-166 (166Ho), yttrium-90 (90Y), and rhenium-186/rhenium-188 (186Re/188Re) (Watanabe et al. 1999; Jay et al. 1998; Nijsen et al. 1999; O’Donnell and McGinity 1997; Mumper and Jay 1999).
11.8 Radionuclide Used for Treatment of HCC
In this modality, 131I-labeled lipiodol, 90Y microspheres, 166Ho microspheres, and 188Re-labeled lipiodol and microspheres are the radiopharmaceutical agents which have been extensively studied. Physical characteristics of these radionuclide are summarized in Table 11.1.
Table 11.1
Characteristic of key radionuclides used for TART treatment of HCC
Radionuclide/production mode | Emission | Half-life (days) | Mean soft tissue penetration depth (mm) | Imaging possibility |
|---|---|---|---|---|
166Ho/Reactor | β−, γ | 1.2 | 1.23 | Yes |
131I/Reactor | β−, γ | 8.04 | 0.4 | Yes |
188Re/Reactor | β−, γ | 0.709 | 4.0 | Yes |
90Y/Reactor | β− | 2.7 | 3.0 | No |
11.9 Radioisotopes for TART
11.9.1 Iodine-131-Lipiodol
Traditionally, 131I was the first beta-emitting radioisotope which had been used and shown to have some efficacy for TART, and a large number of studies using 131I lipiodol for the treatment of unresectable HCC have been reported (Kooijman et al. 2000; Raoul et al. 1994; Bhattacharya et al. 1995; Risse et al. 2000, 2006; Boucher et al. 2003, 2007; Kanhere et al. 2008; Chua et al. 2010). This agent is commercially available as Lipiocis® (CIS Bio International, Gif sur Yvette, France). Lipiodol is an iodinated ethyl ester of the naturally occurring fatty acids of poppy seed oil containing 38 % of iodine by weight which had been used earlier as a contrast medium for the X-ray detection of HCC which is sequestered and remains in these tumors for a longer period compared to normal liver or other tissues. The 131I is chemically attached to the naturally occurring Lipiodol isomers. In order to preclude the toxicity of 131I, it is essential to block the thyroid gland with perchlorate or Lugol’s solution (iodide) before and after the treatment. The procedure involves a selective hepatic artery catheterization and injection of 2–3 mL of 131I-lipiodol with an activity of 0.9–2.4 GBq in single or multiple administrations (Becker et al. 2008; Bretagne et al. 1998). The cumulative radiation doses delivered to tumors generally range from 10 to 260 Gy. The response of HCC and therapeutic outcome is very much dependent on the tumor size as well as the activity levels delivered for treatment. The required therapy activity for the intended tumor dose is calculated for a given tumor mass according to size. There is also a role for 131I lipiodol as an effective therapy option therapy in patients with portal vein thrombosis (Raoul et al. 1994, 2009). One of the main limiting factors for broader use of 131I-lipiodol is the requirement of long period for patient isolation because of the long 8.02-day half-life of 131I. No significant tumor reduction is usually seen in single study treatment of liver metastases. In order to achieve optimum response, multiple sessions may be required. Some authors have expressed their concern about hypothyroidism (Toubeau et al. 2001).
11.9.2 90Y-Labeled Agents
Once 90Y-microspheres became available, and then later when they emerged as approved clinical products, TART with two 90Y-labeled agents emerged as the mainstream treatment modality. The two commercially available 90Y-labeled products are TheraSphere® and SIR-Spheres®. The TheraSphere product consists of small glass particles (MDS Nordion, Ottawa, ON, Canada) and the SIR-Spheres microspheres are fabricated from resin (Sirtex Medical, Sydney, Australia). The TheraSphere® product consists of 90Y embedded into non-biodegradable glass microspheres of 25 μm diameter and have been recently approved by the US Food and Drug Administration (FDA) for treatment of unresectable HCC. SIR-Spheres® consist of biocompatible resin-based microspheres containing 90Y. SIR-Spheres use was granted approval for metastatic colorectal cancer in 2002 (Fig. 11.2). The characteristics of these two preparations are summarized in Table 11.2.
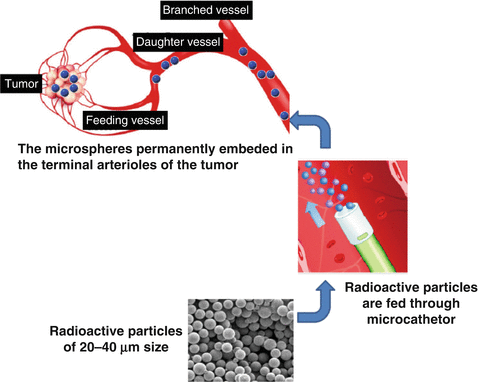

Fig. 11.2
Illustration of intra-arterial release of radioactive microspheres in a hepatic artery
Table 11.2
Comparison of commercially available 90Y-microspheres used for treatment of HCC
Characteristics | SIR-Spheres® | TheraSphere® |
|---|---|---|
Material | Resin | Glass |
Particle size (μm) | 20–60 | 20–30 |
Specific gravity | Low (<1.8) | High (>2) |
Activity per sphere (Bq) | 40–70 | 2,500 |
Maximum prescribed activity (GBq) | 3 | 20 |
Average activity (GBq) | 2 | 5 |
Average dose rate | 1.8 μGy/h, with a maximum of 16 μGy/h | 1.9 μGy/h, with a maximum of 12 μGy/h |
Particles per administration | 20–40 million | 1–4 million |
Regulatory approval | US FDA approval 1999 (colorectal) | US FDA approval 2002 (HCC) |
SIR-Spheres® are used for the treatment of unresectable metastatic liver tumors from primary colorectal cancer, with adjuvant intrahepatic artery chemotherapy of floxuridine. The indication for use of TheraSpheres® is for irradiation treatment or as a neoadjuvant to surgery or transplantation in patients with unresectable hepatocellular carcinoma, who qualify for appropriate hepatic arterial catheter placement. Both glass and resin microspheres produce heterogeneous high-dose regions in the tumor. After injection, the microspheres deliver over 95 % of their total radioactive dose over a period of 2 weeks. Patients typically receive only two treatments to each lobe of the liver, given at approximately 2 months intervals. Detailed accounts of the recommended clinical procedures and patient indicators have been well studied (Murthy et al. 2005; Lau et al. 2004; Uthappa et al. 2011; Kennedy et al. 2012; Kao et al. 2012; Wang et al. 2010; Iñarrairaegui et al. 2010; Hendlisz et al. 2010; Deleporte et al. 2010; Salem and Thurston 2006). Use of these agents is considered as a safe and efficient treatment modality in salvage therapy of colorectal cancer metastatic to the liver and in unresectable HCC when selection criteria are applied strictly, particularly in terms of liver function tests. Treatments have been shown to have good clinical outcome and to increase survival and are approved as definitive therapy, as abridge to transplantation and as downstaging to surgery.
11.9.3 Rhenium-188 Lipiodol/Microspheres
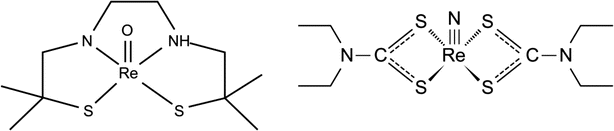
In addition to the routine use of the two commercially available 90Y products, a large number of clinical trials have also been reported using 188R-labeled products combined with Lipiodol for the TART application, because of the ready, in-house, on-demand availability of 188Re from the 188W/188Re radionuclide generator system (Chap. 7). A number of embolic platforms of 188Re such as glass microspheres (Chang and Lin 2007), human serum albumin microspheres (HSAM) (Wunderlich et al. 2005), poly-(l-lactide) (PLA) microspheres (Saatchi and Hafeli 2009), and two Lipiodol complex agents have been studied for their possible use for tumor treatment in inoperable HCC (Andreana et al. 2012). Among these, 188Re 4-hexadecyl-1, 2,9,9-tetramethyl-4,7-diaza-1,10-decanethiol (HDD)-labeled iodized oil (Fig. 11.3) has received maximum attention (Kumar et al. 2007; Sundram et al. 2004; Bernal et al. 2007, 2008; Lambert et al. 2004, 2005; Liepe et al. 2007). Rhenium-188 has obvious advantages over use of 131I due to lower γ emission energy, greater β− penetration, and reduced cost. In addition, the in-house availability of the 188W/188Re radionuclide generator for on-demand preparation of these therapeutic agents was also an important factor. These two lipophilic 188Re-labeled agents (Fig. 11.3) have high miscibility with lipiodol. The resulting quantity of the 188Re HDD iodized oil (lipiodol) administered is based on the radiation absorbed dose (RAD) to critical organs, which is calculated after trans-arterial administration of test dose of the radioconjugate (Kumar et al. 2007). The limited availability as well as the high cost of 188W/188Re generator emerged as the major road block toward its wide-scale applicability. The requirement of greater patient dose due to its shorter half-life is also an impediment. An International Atomic Energy Agency (IAEA)-sponsored multicenter study using intra-arterial 188Re lipiodol for the treatment of inoperable HCC showed safety and efficacy of this radioconjugate Bernal et al. 2007, 2008).