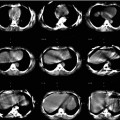
Fig. 12.1
Gastroduodenal accumulation of 99mTc-MAA (yellow arrows, b–d) in a patient with colorectal carcinoma, not definable on planar image (a)
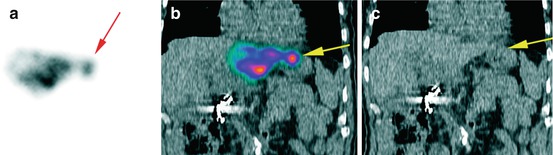
Fig. 12.2
Focally increased gastric uptake of 99mTc-MAA after injection into the left hepatic artery in a 67 y/o patient with colorectal carcinoma (yellow; b and c), not differentiable from a liver uptake on planar image (red arrow; a)
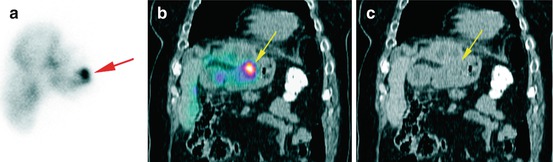
Fig. 12.3
Focally increased gastric uptake of 99mTc-MAA after injection into the right and left hepatic arteries in a 66 y/o patient with cholangiocellular carcinoma (yellow; b and c), not exactly differentiable from a liver uptake on planar image (red arrow; a)
12.4.2 99mTc-MAA Accumulation in the Gallbladder
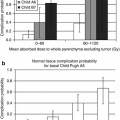
Radiation cholecystitis is usually subclinical but it is associated with post-procedure morbidity requiring surgical intervention in about 1 % of patients [27]. The gallbladder can be evaluated more precisely with SPECT/CT compared to SPECT. In 8–12 % of patients, a 99mTc-MAA accumulation in the gallbladder can be detected by SPECT/CT [15–17]. The approach in cases showing 99mTc-MAA accumulation in the gallbladder is controversial from no therapy plan changing and prophylactic antibiotic therapy to cholecystectomy prior to RE [15–17]. One strategy to prevent the spheres from reaching the gallbladder and the resulting radiation-induced cholecystitis may be the placement of the catheter distal to the cystic artery. However, this method is only reasonable if it does not result in an inadequate in target distribution of microspheres. The alternative approach could be the prophylactic embolisation of the proximal cystic artery with either absorbable gelatine sponge pledgets or fibred microcoils immediately before radioembolisation, which has been shown to be safe and feasible [28]. The intensity of tracer uptake in the gallbladder could also be a helpful guide for making the decision, however, without established consensus [16].
12.4.3 99mTc-MAA Accumulation in the Anterior Abdominal Wall (AAW)
Tc-MAA uptake in the anterior abdominal wall (AAW) has been described as a sign of patent hepatic falciform artery (HFA) [29] with a reported incidence of up to 13 % [16, 30–33]. The HFA arises as a terminal branch of the middle or left hepatic artery. It runs within the hepatic falciform ligament with the umbilical vein, provides partial blood supply around the umbilicus and communicates with branches of the internal thoracic and superior epigastric arteries [34]. It is known that influx of chemoembolic agents into the HFA can cause supraumbilical skin rash, epigastric pain and skin necrosis. Normally, a patent HFA appears on planar 99mTc-MAA scans as an elongated tracer accumulation in the mid-abdomen, which could be anatomically better localised with SPECT. SPECT/CT can increase the detection rate of such uptake (Fig. 12.4) [16, 31] and can additionally detect the falciform ligament, especially after multiplanar reconstruction in the coronal view, which increases diagnostic accuracy [31]. Generally, 99mTc-MAA uptake via the HFA does not result in diagnostic problems except for patients with umbilical hernia [16]. As for all other extrahepatic arteries, we recommend the prophylactic embolisation of HFA in the test angiogram as well as an angiographic reassessment in the patients with 99mTc-MAA accumulation in AAW prior to the application of 90Y microspheres (Figs. 12.5 and 12.6). However, the post-selective internal radiotherapy side effects in patients with tracer accumulation in AAW on 99mTc-MAA scan are neither common nor severe [16, 31, 33]. Leong et al. reported one case of self-limiting radiation dermatitis caused by shunting of 90Y microspheres to the AAW via a patent HFA [35]. In a recently published study, a HFA was identified in 99mTc-MAA SPECT/CT in 16 patients who did not undergo coil embolisation during treatment; only one patient complained of abdominal pain for 48 h, without presenting skin lesions [31]. Thus, there is no absolute need for prophylactic embolisation of the HFA or a treatment plan modification if the HFA is not detectable in an angiography session. Otherwise, being informed about such accumulations can avoid performing multiple and unnecessary endoscopies to detect GI ulcers in patients with unexpected abdominal pain [16] without any detectable gastroduodenal 90Y uptake in post-therapeutic BS scan.
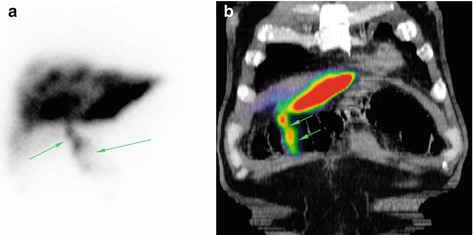
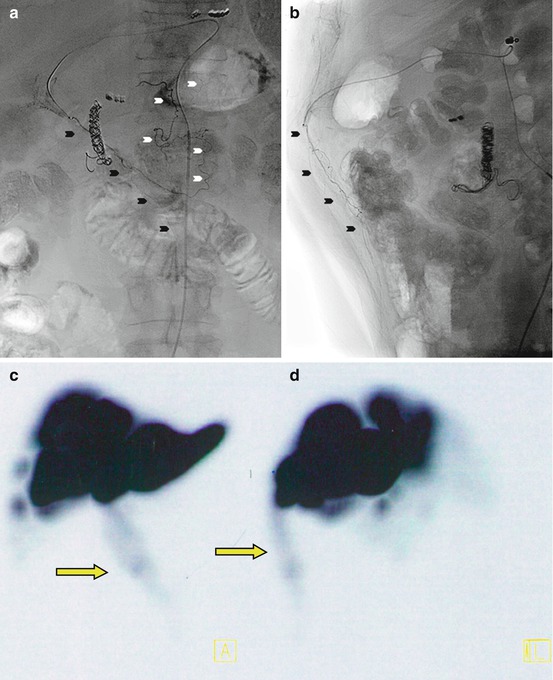
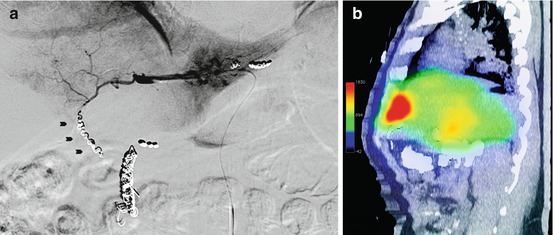

Fig. 12.4
(a) Planar scan showing 99mTc-MAA accumulation in the anterior abdominal wall (yellow arrows), indicating a patent hepatic falciform artery. (b) Coronal 99mTc-MAA SPECT/CT image in the same patient

Fig. 12.5
Selective catheterisation of hepatic falciform artery (black arrowheads) in posterior-anterior (a) and lateral view (b) demonstrating the characteristic filling as it travels through the falciform ligament, terminates in the anterior abdominal wall, and anastomoses via collaterals (white arrowheads) with the superior epigastric vessels (c, d). Correspondent 99mTc-MAA SPECT scan maximum intensity projection coronal view (c) and sagittal view (d) show a tracer accumulation in anterior abdominal wall (yellow arrow) (With kind permission from Springer Science + Business Media: [38], Fig. 2)

Fig. 12.6
(a) Injection in the left hepatic artery after selective embolisation of hepatic falciform artery (black arrowheads) of the same patient showed in Fig. 12.5. (b) Multiplanar reconstructed sagittal bremsstrahlung SPECT/CT 24 h after radioembolisation showed no extrahepatic tracer accumulation in anterior abdominal wall (With kind permission from Springer Science + Business Media: Ahmadzadehfar et al. [48], Fig. 3)
12.4.4 Other Types of Extrahepatic 99mTc-MAA Uptake
Recently, Lenoir et al. reported tracer uptake in the hepatic artery in 6.6 % of patients [16], which had no impact on patient management. This arterial uptake is likely due to the impaction or aggregation of MAA by arterial microlesions caused by long and complicated angiography procedures or when arteries are weakened by previous procedures [16]. Tracer accumulation in the coil embolisation site can be detected only by SPECT/CT and the detection rate has been reported to be less than7 % [15, 16]. Two reasons for this type of accumulation were described [15]. First, small aberrant vessels ending in this region, which should be found and coil embolised before RE or injection of microspheres, should be performed distal to the vessel; a second possible reason is an incompletely embolised vessel. Knowledge of the anatomic region of extrahepatic perfusion may help the radiologist to estimate the origin of the aberrant vessel. The incomplete coil embolised vessels should be embolised immediately before RE [15].
A light focally increased tracer uptake in the spleen has been reported by Ahmadzadehfar et al. in two cases (2.2 %) [15], who also showed 90Y microsphere uptake on BS SPECT/CT [36] without any complications. It seems such tracer accumulations are not harmful. Nevertheless, in such cases the patients should be informed about possible unknown side effects prior to RE. There are also some unusual sites of extrahepatic shunts that seldom occur, such as tracer uptake in the left side of the diaphragm [15, 22], distal of the oesophagus [22, 37], in the paracaval lymph nodes [22], in the pericardium or focally increased uptake in the pulmonary left lower lobe. In such cases, it is recommended to coil embolise the aberrant vessels.
12.5 99mTc-MAA SPECT/CT in the Evaluation of Intrahepatic Tracer Distribution and Its Significance in Therapy Planning
The aim of RE is to treat the complete tumoural area and to avoid, as much as possible, the delivery of particles into the healthy liver tissue. Although intrahepatic accumulation of 99mTc-MAA – especially in cases of single liver tumours – could be acceptably assessed by planar scan and SPECT imaging, only SPECT/CT can provide a precise evaluation of tracer distribution in the patients with multiple liver tumours and metastases, e.g. 99mTc-MAA uptake in the tumour thrombus of the portal vein, which is more commonly seen with HCC [22], can be detected only by SPECT/CT. A portal tumour thrombus may be seen on SPECT/CT as a continuous area of activity extending from the tumoural lesion in the liver parenchyma into the portal system [22] and it suggests that the tumour thrombus is supplied with blood through neovascularisation from the hepatic arteries, as is the tumour itself [22]. 99mTc-MAA uptake in the tumour thrombus may be a predictor of a favourable response to RE. In a study by Garin et al. [38], 92 % of responding patients with portal vein thrombosis (PVT) showed 99mTc-MAA uptake in the PVT on SPECT/CT images and only in one non-responder with a large tumour was tracer uptake seen in the PVT [38]. Although a positive correlation between the amount of tumoural 99mTc-MAA uptake and treatment response and its significance in the pre-therapeutic dosimetry has been shown, this issue should still be studied with more patients in prospective settings (Fig. 12.7). Flamen et al. treated ten patients with colorectal cancer. They showed a cut-off value of 1 for the 99mTc-MAA uptake ratio could predict a significant metabolic response with a sensitivity of 89 %, a specificity of 65 %, a positive predictive value of 71 % and a negative predictive value of 87 % [39]. Garin et al. in a study of 36 patients with HCC using 90Y-glass microspheres showed that quantitative 99mTc-MAA SPECT/CT is predictive of response, progression-free survival and overall survival. They suggested that using tumour dosimetry based on 99mTc-MAA SPECT/CT imaging could allow an adaption of the therapeutic planning and the activity to be administered [38, 40]. In a recently published paper by the same group, they reported that 99mTc-MAA SPECT/CT predicted response with a sensitivity of 100 % and overall accuracy of 90 % in 71 HCC patients [41]. In contrast, Van de Wiele et al. reported in a study of 13 patients with liver metastases from different origins that the percentage of 99mTc-MAA uptake by lesions was not significantly different between responding and non-responding lesions [24].
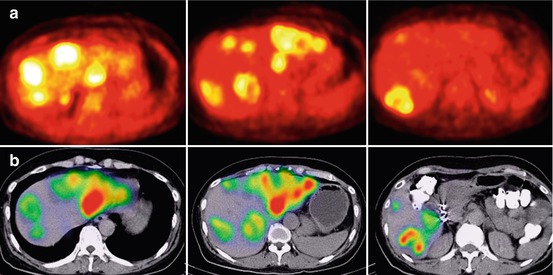

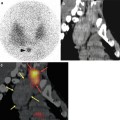
Fig. 12.7
(a) Liver 18F-FDG PET of a patient with liver metastases of colorectal carcinoma shows high FDG accumulation in multiple metastases. (b) 99mTc-MAA SPECT/CT of the same patient shows high tumour accentuated tracer accumulation without any relevant uptake in the healthy liver tissue. The patient showed a near complete response after treatment with resin microspheres
Nevertheless, an absence of 99mTc-MAA uptake in one part of a tumour or one or more liver segments with metastases, detected on SPECT/CT images, is an important issue (Fig. 12.8). A tumour may have an unidentified accessory arterial blood supply or may have parasitised nearby arteries [22]. The liver segments that do not show any 99mTc-MAA uptake because of accessory arterial blood supply or parasitised arteries normally cannot also be a target for 90Y microspheres. A high and diffuse accumulation of 99mTc-MAA in the healthy liver tissue is also an important issue. A high radiation dose to the healthy liver tissue can increase the probability of developing a REAILD. The liver vascular mapping with 99mTc-MAA SPECT/CT imaging makes it possible to evaluate the tumoural and non-tumoural blood supplying vessels, which is of importance for avoiding unnecessary radiation exposure to the nontarget liver tissue and for achieving the tumoural area as much as possible. Sometimes it just needs a tracer application more distally; however, it is not always a straightforward issue and needs special interventional approaches for better targeting.
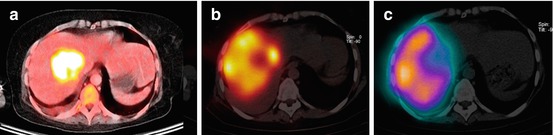

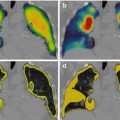
Fig. 12.8
(a) High FDG uptake in a single liver metastasis in a patient with sarcoma. (b) 99mTc-MAA SPECT/CT showed no tracer uptake in the metastasis. (c) Bremsstrahlung SPECT/CT after therapy with resin microspheres showed also no 90Y microsphere uptake in the metastasis. A progression disease in follow-up confirmed these imagings
12.5.1 Detecting Blood Vessels Feeding the Tumour and Selective Administration into These Vessels
In the case of a discrepancy between the segmental distribution of 99mTc-MAA and the intended vascular territory to be treated, the angiograms should be carefully reviewed trying to find the reason for such discrepancies. It could be because of a tracer injection distal to a branching point that excludes part of the tumoural area [22], but sometimes the accessory arterial blood supplying vessels or parasitised arteries are the cause of such discrepancies [22]. In such cases, the test angiogram should be repeated and 99mTc-MAA should be injected selectively into the tumour-feeding arteries followed by SPECT/CT imaging for confirmation of exact targeting.
12.5.2 Flow Redistribution
Accessory or replaced hepatic arterial branches are a relatively frequent finding. The occlusion of accessory or aberrant arteries is supposed to result in flow redistribution from two (or multiple) arteries to a single artery. Thus, the infusion of 90Y microspheres for one liver lobe can be accomplished via one artery (rather than by two or several arteries). In 89–95 % of cases after occlusion of the accessory or aberrant arteries, a flow redistribution is detectable (angiographic or on 99mTc-MAA or on BS SPECT/CT) (Fig. 12.9) [27, 42, 43




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree