Introduction
Historically, radionuclide lung imaging was used mainly for the diagnosis of pulmonary embolism (PE) and lung function. The advent of fluorodeoxyglucose (FDG) positron emission tomography (PET) computed tomography (CT) scanning, with its integration of both functional and anatomic information into coregistered datasets, has heralded a new age of radionuclide imaging for lung cancer evaluation and staging. Expanded applications of FDG PET/CT now also include non-neoplasm imaging. Although the interpretation of nuclear studies remains primarily qualitative, an important advantage of modern nuclear medicine instrumentation and computing power is the availability of quantitative imaging analysis, which can supplement and support qualitative observations.
Part I: Principles of Pulmonary Scintigraphy
Pulmonary scintigraphy—or V/Q scanning—allows for the noninvasive interrogation of two key aspects of lung function: bronchopulmonary ventilation and pulmonary vasculature perfusion. The underlying principle is that inhaled radioactive gases or radioaerosols and intravenous radiolabeled small particles will distribute according to air and blood flow, respectively. Radiotracer distribution creates a physiologic map of ventilation and perfusion; areas of low radiotracer distribution suggest an obstructed flow, shunting, or surgical defect.
Historically, pulmonary scintigraphy has been the most important diagnostic tool for evaluating PE. In the modern era, CT has become the first-line imaging modality for detecting and monitoring most thoracic pathology; however, pulmonary scintigraphy continues to provide unique physiologic information and remains an indispensable adjunct to anatomic imaging.
Radiopharmaceuticals
Ventilation
Ventilation imaging can be performed with radioactive gases or radioaerosols. Only xenon-133 ( 133 Xe), a radioactive gas, and technetium-99m ( 99m Tc) diethylenetriamine pentaacetic acid ( 99m Tc-DTPA), a radioaerosol, are currently available in the United States. Comparative studies between 133 Xe and 99m Tc-DTPA have not shown significant differences in overall accuracy. The choice of agent varies by institution and depends largely on available equipment and physician preference.
Xenon-133
133 Xe is a noble gas produced by fission of uranium-235 ( 235 U) in a nuclear reactor. 133 Xe has a half-life of 5.3 days. The principle photon energy is 81 keV. Although 133 Xe is partially soluble in blood, it clears rapidly from the blood as it is exhaled, resulting in a biologic half-life of 30 seconds by current imaging standards. A typical adult dose of 133 Xe for a ventilation scan is 10 to 30 mCi.
Technetium-99m DTPA
99m Tc-DTPA is produced by nebulizing its liquid form into a fine mist that is then filtered through a settling bag into a microaerosol. Once inhaled, larger particles (>2 µm) deposit centrally in the trachea and pharynx; smaller particles (<0.5 µm) deposit in the distal tracheobronchial tree. Less than 10% of the aerosolized radioisotope is deposited within the lungs. Once in the lungs, 99m Tc-DTPA diffuses across the alveolar membrane through the interstitium into the capillaries and is ultimately excreted by the kidneys. The biologic half-life of DTPA is 60 to 90 minutes, whereas the physical half-life of 99m Tc is 6 hours. Airway turbulence caused by any obstructive lung disease results in more central deposition of particles, potentially compromising visualization of peripheral lung. The advantages of 99m Tc-DTPA are that it is widely available and inexpensive and has a 140-keV photopeak, ideal for gamma camera imaging. A typical adult dose of nebulized 99m Tc-DTPA is 30 mCi.
Perfusion
The goal of perfusion lung scanning is to distribute the radiopharmaceutical to pulmonary segments according to relative blood flow. This can be achieved using radiolabeled particles just larger than the diameter of capillaries (7–10 µm). Once injected intravenously, the particles travel through the right atrium, right ventricle, and then the pulmonary vascular bed, where they become trapped in the precapillary arterioles. Pulmonary segments with decreased or absent blood flow have reduced radiopharmaceutical delivery and trapping, resulting in a defect.
99m Tc-macroaggregated albumin (MAA) is the most common radiopharmaceutical in current use. MAA is prepared by heat denaturation of human serum albumin. 99m Tc-MAA is prepared by adding 99m Tc-pertechnetate to a commercially available MAA kit. Of the radiolabeled particles, 90% measure between 10 and 100 µm. Particles larger than 150 µm should not be injected because they can obstruct arterioles. Typically, 200,000 to 500,000 particles are injected, and less than 0.1% of capillaries are temporarily occluded. A minimum of approximately 60,000 99m Tc-MAA particles must be injected to ensure reliable count statistics and image quality. The standard dose of 99m Tc-MAA is 4 mCi, although larger doses are administered for heavier patients.
The biologic half-life of MAA particles in the lung is 2 to 12 hours. The MAA degrades over time and passes through the alveolar capillaries into the systemic circulation, where it is phagocytized by the reticuloendothelial system.
Scan Techniques
Xenon-133 Ventilation Scanning
The patient is initially fitted with an airtight face mask. 133 Xe is inhaled from a closed shielded system. The patient is instructed to take a large breath and to hold it for as long as possible. A posterior projection 30-second wash-in or initial breath image of the lungs is obtained. The patient then rebreathes an air– 133 Xe mixture. After 3 to 5 minutes of rebreathing, posterior, left posterior oblique, and right posterior oblique equilibrium images are obtained for 60 seconds each. The air– 133 Xe mixture can be titrated with oxygen as needed. In the washout phase, the patient exhales the 133 Xe mixture into a charcoal trap. Serial posterior and lateral oblique 60-second washout images are obtained over a 5-minute interval. If possible, all images should be performed with the patient in an upright position. 133 Xe ventilation scanning should be performed in a negative pressure room with safe external ventilation.
Technetium-99m DTPA Ventilation Scanning
The patient inhales 99m Tc-DTPA microaerosol for 3 to 5 minutes. This part of the examination should be performed with the patient in the supine position to minimize the normal apex to base gravity-induced ventilation gradient. After inhaling 99m Tc-DTPA, the patient is imaged, preferably upright, in the anterior, posterior, right lateral, left lateral, right posterior oblique, left posterior oblique, right anterior oblique, and left anterior oblique positions for 500,000 counts each. One important advantage of 99m Tc-DTPA ventilation scanning is that matching imaging projections as for perfusion scanning can be obtained. The exhaled aerosol is trapped in a filter that is stored to allow for decay until safe for disposal.
Technetium-99m MAA Perfusion Scanning
99m Tc-MAA particles should be resuspended prior to injection by gentle agitation. The patient is injected in the supine position while taking slow deep breaths to facilitate lung expansion and reduce atelectasis. Blood should not be drawn into the syringe because aspirated blood may form clots, which become labeled by the 99m Tc-MAA.
The patient is preferably imaged in the upright position using a large field of view, all-purpose gamma camera. Standard views in the anterior, posterior, right lateral, left lateral, right posterior oblique, left posterior oblique, right anterior oblique, and left anterior oblique positions of at least 500,000 counts each are obtained.
Importance of Sequence of Imaging
133 Xe ventilation scans should be obtained before perfusion lung scans because Compton scatter from the higher energy 99m Tc-MAA photons (140 keV) down-scatters into the region of the 81-keV photopeak of the 133 Xe and would thus interfere with ventilation images.
A 99m Tc aerosol ventilation lung scan should be performed after the perfusion lung scan. A standard dose of 99m Tc-MAA is used, followed by a larger dose of 99m Tc-DTPA (30 mCi); the rationale is that the higher activity of the 99m Tc-DTPA scan will overwhelm any abnormalities present on the initial 99m Tc-MAA scan. The historical practice of performing 99m Tc-DTPA ventilation scan first, followed by 99m Tc-MAA perfusion imaging, is no longer considered adequate.
Patient Preparation
A standard chest radiograph, preferably in both posterior-anterior and lateral projections, should be obtained within 24 hours of pulmonary scintigraphy. CT can substitute for chest radiography.
In patients with acute and symptomatic obstructive lung disease, bronchodilator therapy could be considered prior to lung scanning to reduce ventilation abnormalities that would result in a nondiagnostic study.
Contraindications to Pulmonary Scintigraphy
There are few absolute contraindications to the ventilation or perfusion phases of pulmonary scintigraphy. Allergy to human serum albumin products is a contraindication to perfusion imaging, but occurs very rarely.
A few conditions warrant precautionary measures during perfusion imaging. Patients with pulmonary hypertension have reduced pulmonary reserve, and patients with right-to-left shunts are at risk for intracranial microemboli. As such, these patients should be injected with fewer particles. The standard 4-mCi dose should be administered but with fewer particles, typically less than 150,000; this will often require reconstituting a fresh MAA kit with higher than standard 99m Tc activity per particle. Alternatively, if lower than standard activity is administered, each perfusion view can be imaged for a longer time interval to achieve comparable counting statistics.
Children should also receive a reduced number of particles because they have fewer pulmonary arterioles. Specific guidelines for dosing children have been published by the European Society of Nuclear Medicine. A minimum of 10,000 particles should be administered.
Clinical Applications for Pulmonary Scintigraphy
Acute Pulmonary Embolism
The most important application for pulmonary scintigraphy is the detection of acute pulmonary emboli. Although CT has largely replaced pulmonary scintigraphy as the first-line diagnostic tool for diagnosing acute PE, pulmonary scintigraphy remains the modality of choice in patients for whom CT angiography is contraindicated, usually those with impaired renal function and allergy to iodinated contrast.
Comparison of Diagnostic Performance of Pulmonary Scintigraphy to CT Angiography
According to the original Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED I) trial data, a high-probability V/Q scan interpretation was associated with a sensitivity of 41% and specificity of 97% for detecting PE when compared to a pulmonary angiogram as the reference standard. If a scan was interpreted as either high or intermediate probability, the sensitivity improved to 82%, with a specificity of 52%. If a scan was interpreted as any category other than normal (including low probability), the sensitivity was 98%.
In the PIOPED II trial, comparing CT angiography to a composite standard, CT angiography was 83% sensitive and 95% specific in detecting PE. Combining CT venography increased the sensitivity from 83% to 90%. In practice, the diagnostic performance of any modality evolves with usage pattern and advances in technology. Diagnostic performance is also highly dependent on an individual radiologist’s practice experience. As CT angiography continues to replace pulmonary scintigraphy as the preferred diagnostic study to diagnose PE by clinicians and radiologists, comfort with and proficiency at interpreting V/Q scans declines. It should be noted that a key advantage to CT angiography is that it can reveal coexisting or alternative causes that could explain a patient’s symptoms.
As screening tools, both a diagnostic quality CT angiogram showing patent pulmonary arteries and normal or near-normal V/Q scans have comparable and high negative predictive values exceeding 95% for excluding PE.
Chronic Pulmonary Embolism
A chronic PE represents a distinct clinical entity from acute PE. Pulmonary scintigraphy is the diagnostic modality of choice for the diagnosis of chronic thromboembolic disease. Direct comparisons of multidetector CT and pulmonary scintigraphy have shown higher sensitivity (96%–97% vs. 51%). The diagnostic criteria for a chronic PE are identical to those for an acute PE.
V/Q Scans
Quantitative V/Q Scan
Ventilation or perfusion can be quantified by drawing regions of interest around the upper, mid, and lower zones of each lung and calculating each zone’s contributions to total lung activity using geometric mean calculations. Generally, this technique is used to estimate pulmonary function in a patient undergoing lung resection for lung carcinoma. The postoperative FEV 1 (forced expiratory volume in 1 second) can be estimated by multiplying the preoperative FEV 1 by the fractional perfusion supplying the nonresected lung regions. These data are important because many lung cancer patients have coexisting chronic lung disease. In the evaluation of the posttransplant lung, altered perfusion distribution or the development of matched ventilation perfusion defects may indicate transplant dysfunction and rejection.
Normal V/Q Scan
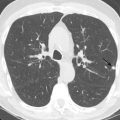
A normal V/Q scan shows homogeneous and matched distribution of radiotracer on ventilation and perfusion images that outlines the shape of the lungs on radiograph ( Fig. 12.1 ). There is expected cardiac, and sometimes hilar and aortic, impressions. A subtle base-to-apex gradient may be present because there is more basilar than apical lung parenchyma.


The 133 Xe parenchyma ventilation scan includes three phases—initial breath or wash-in, equilibrium, and washout. The wash-in images are often noisy because of relatively poor count statistics. Poorly ventilated lung will manifest as regions of low activity, which may fill in on equilibrium phase images. The distribution of 133 Xe activity on the equilibrium image represents aerated lung volume. The 133 Xe normally clears from the lungs within 3 minutes. Because the lung bases are better ventilated than the apices, the 133 Xe washes out of the bases faster than at the apices in a normal patient. Focal or diffuse retention of xenon is indicative of obstructive lung disease.
A normal 99m Tc-DTPA aerosol scan often demonstrates activity within the trachea and the main stem bronchi in patients with obstructive lung disease. Swallowed 99m Tc-DTPA aerosol activity can outline the esophagus and the stomach.
Approach to Interpreting the Abnormal V/Q Scan
The basis of pulmonary scintigraphy or V/Q scanning for PE is to detect a mismatch of pulmonary ventilation, which is relatively preserved, and pulmonary arterial perfusion, which is obstructed in the lung distal to a pulmonary artery embolus. Fundamentally, the interpretation of a perfusion defect rests on whether there is a matched defect on a ventilation image or radiograph that suggests an alternative explanation for the perfusion abnormality other than PE; if not, the scan has to be considered suspicious.
One consideration to bear in mind when comparing V/Q abnormalities to a standard radiograph is that V/Q images are obtained during tidal breathing, whereas chest radiographs are obtained at maximal inspiration. As a result, the lungs are smaller on the lung scan images than on radiographs. The reader should account for this difference in technique when deciding whether abnormalities are “matched” in size and shape.
Established Criteria for Interpreting V/Q Scans
The first structured schema was published by to standardize the interpretation of V/Q scans into low-, intermediate-, and high-probability categories. This schema was refined through the PIOPED study, a multiinstitutional trial designed to define evidence-based risk categories for PE. The PIOPED criteria stratified the risk of PE based on the number, size, shape, and distribution of perfusion defects and associated ventilation and radiographic findings. Based on these criteria, 13% of trial subjects had high-probability V/Q scans, 39% had intermediate, 34% had low, and 14% had normal or near-normal scans. A low-probability study conferred a less than 20% risk of PE, an intermediate-probability study conferred a 20% to 80% risk, and a high-probability study conferred a greater than 80% risk.
The PIOPED system posed several challenges for guiding clinical management because a risk of up to 20% for a low-probability scan was unacceptably high, and a range of 20% to 80% probability in the intermediate category was considered too broad to be prescriptive. Numerous revisions to the original PIOPED criteria have been proposed, including the modified PIOPED II criteria, a simplified classification system derived from the more recent PIOPED II data. The goal was to shift V/Q scan interpretations to high- and very low-probability categories and to add a nondiagnostic category. Specifically, the updated risk categories include the following:
- 1.
Normal—that is, no perfusion defect (essentially excluding PE, irrespective of ventilation scan findings)
- 2.
High probability of pulmonary emboli (positive predictive value [PPV], 87%) or
- 3.
Very low probability (PPV, 6%)
| FINDING | CATEGORY |
|---|---|
| Two or more large segmental defects Perfusion defect much larger than ventilation and radiographic abnormality | High probability |
| Nonsegmental perfusion abnormalities Perfusion defects smaller than corresponding radiographic abnormality Solitary triple-matched defect in the mid and upper lung zones One to three segmental perfusion defects (<25% segment) Moderate to large pleural effusion | Very low probability |
| All other findings | Nondiagnostic (low or intermediate probability) |
Causes and Implications of Diffusely Heterogeneous Pulmonary Perfusion Pattern
A diffusely heterogeneous pattern of pulmonary perfusion on a 99m Tc-MAA perfusion scan can result from both technical and clinical factors. The most important technical factor is insufficient counts, usually related to insufficient particles due to partial extravasation of the dose. A low counting rate in the lungs and high counting rate at the injection site confirm a partially extravasated dose. Clinical entities that can contribute to a heterogeneous perfusion pattern include lymphangitic carcinomatosis and primary pulmonary hypertension. The distinction between primary pulmonary hypertension and pulmonary hypertension secondary to chronic thromboembolic disease is important because the latter may be treatable by anticoagulation and/or surgical thromboendarterectomy.
Reverse Mismatch
Reverse ventilation-perfusion mismatches are ventilation defects without corresponding perfusion abnormalities. Reverse mismatches result from a failure of vasoconstriction that is usually induced by hypoxia in unventilated lung. This results in a functional right-to-left shunt because poorly oxygenated blood is never reoxygenated before returning to the systemic circulation. Mucus plugging is the most common cause of a reverse mismatch.
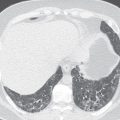
Examples are as follows. Typically, pulmonary emboli present as multiple segmental or wedge-shaped perfusion defects, with normal or near-normal ventilation and a clear radiograph ( Fig. 12.2 ). It should be emphasized that pulmonary scintigraphy cannot confidently estimate the age of pulmonary emboli and therefore cannot reliably distinguish acute from chronic abnormalities.


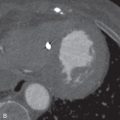
While in the appropriate clinical setting, acute pulmonary emboli are the most common cause for segmental mismatched defects, but they are not the only cause. The most common alternative diagnosis is lung cancer. Pulmonary vasculature is preferentially occluded by mass effect from a mediastinal tumor or lymphadenopathy compared to the more rigid bronchi ( Fig. 12.3 ). Old emboli are another consideration in the absence of prior studies for comparison. A list of additional differential diagnoses, most of which are exceedingly rare, is shown in Box 12.1 .