Since the introduction of spinal cord stimulation in 1967, the therapy has become a cornerstone in the treatment of several chronic pain syndromes, including but not limited to: postlaminectomy syndrome, lumbar radiculopathy, complex regional pain syndrome and diabetic peripheral neuropathy. This article aims to examine the methodology and practical considerations involved in thoracolumbar spinal cord stimulation implementation, emphasizing procedural techniques and critical criteria for selecting patients to achieve optimal outcomes and minimization of complications.
Introduction
Since the first description of “Dorsal Column Stimulation” by Shealy et al. in 1967, the concept of utilizing electrical current to modulate pain signals via spinal cord pathways has become a mainstay of treatment for various chronic pain conditions. The authors had based their work off of the work previously published by Melzak and Wall in 1965 where they introduced the “Gate Control Theory of Pain”. In this foundational article, the authors posited that the neural cells of the substantia gelatinosa in the dorsal horn play a key role in the regulation and transmission of pain signals propagated centrally. While refined and refuted to varying degrees, this remains a leading theory within pain research.
Spinal cord stimulation was largely paresthesia based, with mapping of paresthesia over areas of chronic pain with radicular pain patterns having the highest rates of success. With paresthesia-based systems, North et al. in 2005 were able to show superiority over reoperation in the setting of what they termed at the time “Failed Back Surgery Syndrome”. Patients in that study demonstrated improvements in pain as well as decreased rates of crossover to reoperation, with a later publication by the same author signaling improved cost effectiveness compared to reoperation.
Subsequent iterations of the technology have led to improvements in outcomes and expanded indications. Beginning in 2015, a shift from paresthesia or “tonic” based systems began after the publication of the SENZA trial comparing a novel high-frequency waveform (10,000 Hz) versus traditional low-frequency programming. The authors showed superiority of the novel waveform in leg pain as well as axial low back pain. Since that time, other programming paradigms have emerged , with continued improvements seen in patient usage and durability of therapy.
Preoperative planning
The preoperative evaluation for spinal cord stimulation (SCS) is of paramount importance. Before undergoing an SCS trial, most patients are required to undergo a comprehensive psychological screening to exclude active alcohol or drug abuse, severe cognitive impairment, unsuitable living conditions, unsupportive environments, and personality disorders. While these factors do not inherently predict poor outcomes, the behavioral assessment serves to address psychosocial issues prior to considering an SCS trial. This process includes recommending treatments for psychological risk factors and specific pain syndromes, as well as evaluating the patient’s personal coping abilities and available support systems.
Obtaining appropriate diagnostic imaging of the lumbar and thoracic spine prior to an SCS trial is essential, as the success of the trial hinges on the proper placement of stimulator leads within the epidural space. This space must be assessed for accessibility and must be free from tumors, vascular malformations, or any other anomalies that would necessitate surgical intervention. Additionally, central canal stenosis must be ruled out, as placing a lead in an area with subclinical stenosis could result in myelopathy, and failure of the trial.
After completing the psychological assessment and reviewing the lumbar and thoracic MRIs, an evaluation for coagulation disorders should be conducted. Patients should be questioned about their use of prescribed blood thinners, as well as over-the-counter anti-inflammatories and supplements, including fish oil, garlic, and vitamin E. It is crucial to have risk versus benefit discussions with the patient’s primary care physician and/or cardiologist regarding the cessation of any prescribed anticoagulation medications. These discussions should include specific recommendations for the duration of medication holds and potential bridging strategies.
Surgical site infections (SSIs) pose significant morbidity and mortality risks, with Staphylococcus species being the most common causative agents, particularly at the electrode implant site. Most contamination stems from the patient’s own skin flora. According to the Neurostimulation Appropriateness Consensus Committee (NACC) Recommendations for Infection Prevention and Management, all remote infections, including dental, skin, and urinary tract infections, must be identified and treated before proceeding with trials or implants. Optimizing glucose control is crucial for diabetics due to their heightened risk of infection, and normalization of their Hemoglobin A1C with their primary care could decrease the risk of SSIs exponentially. Tobacco products should be discontinued for 4 weeks prior to the implant, or patients should use a transdermal nicotine patch during this period. Additionally, patients should decolonize Methicillin-Sensitive Staphylococcus aureus (MSSA) and Methicillin-Resistant Staphylococcus aureus (MRSA) through the application of mupirocin nasal ointment and chlorhexidine baths. To further avoid SSIs, preoperative weight-based antibiotic dosing should be administered within 1 hour prior to surgical incision. Cefazolin is the preferred antibiotic, but if they have an allergy, clindamycin or vancomycin should be used. If hair removal needs are deemed necessary, it should be performed with electric clippers immediately before the trial or implant.
Trial lead placement
After careful review of imaging and preoperative assessments, the patient is scheduled for a trial of spinal cord stimulation prior to implantation. In the vast majority of patients, percutaneous cylindrical leads are placed on an outpatient basis. The purpose of the trial is to ascertain the likelihood of benefit with spinal cord stimulation during activities of daily living. While visual analog scale (VAS) is the most commonly obtained measure of success, measurements of activity, sleep, and mood should also be obtained. Ideally, these measures should be obtained and documented prior to the placement of trial stimulation leads and after completion of the trial.
While historically performed in a hospital setting, in the United States the placement of trial leads has shifted to office-based settings and ambulatory surgery centers over the last 20 years. Regardless whether performed in office or in a facility, trial placement should occur in a sterile environment with utilization of strict sterile technique including skin antisepsis with chlorhexidine and sterile drapes. Preoperative intravenous antibiotics should also be administered prior to starting in accordance with NACC guidelines, as stated above. The use of antibiotics postoperatively remains controversial with most authors arguing against or recommending usage only in select patients. Additionally, the patient should remain awake and conversant during trial lead placement to avoid potential neural injury as well as other complications.
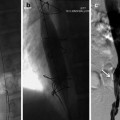
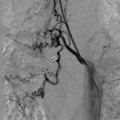
As with most pain procedures, the appropriate usage of image guidance is crucial for successful placement and ultimate successful outcome. Selection of skin entry point and midline epidural access point should be determined preoperatively. While several authors would contend that entry at or below the conus medullaris is the safest entry point, in many patients this is not possible or advisable due to prior surgery or lumbar spinal stenosis at that level. After determination of entry point, skin anesthesia, and puncture should be obtained 1 to 1.5 levels below entry point to allow for a relatively flat entry angle (<45 degrees). A flat entry angle allows for greater steerability of the cylindrical lead, greater chance of successful, posterior, and midline placement. After obtaining access to the epidural via loss of resistance technique, the leads are advanced under fluoroscopic guidance using fine rotational movements to adjust lateral position. Care should be taken to maintain lead position as close as possible to midline to prevent placement in the anterior spinal canal ( Figure 1 ). This procedure is repeated for placement of a second lead. A second lead is considered desirable to obtain additional programming options as well as redundancy.

The final placement level of spinal cord stimulator leads is disease as well as waveform dependent. However, for low back and lower extremity pain most spinal cord stimulator leads are ideally placed midline with distal contact overlying the T7 or T8 superior endplate. Ultimately, during a trial of SCS distal migration of approximately 0.5 vertebral bodies should be expected and considered in final lead placement. Some authors at this point recommend paresthesia mapping to assure coverage of painful areas, however with newer nonparesthesia dependent waveforms, this has become less common. At this point, the Tuohy needles as well as internal stylets are removed. The remaining leads are then secured to the skin with either adhesive or suture and covered with occlusive dressing. The patient is then taken to the recovery area where programming is performed.
At the end of the predetermined trial period, the patient presents to the clinic for removal of trial leads. The device is turned off, the occlusive bandage removed, and gentle traction placed on the stimulation leads until removed intact. A sterile bandage should then be applied. Documentation of trial length, percentage relief, as well as change in activity level, sleep, and mood should be performed. Should the patient meet required payor criteria for successful trial, the patient can then be scheduled for permanent implantation in the operating room ideally no less than 3 to 4 weeks after lead removal.
Permanent implantation
In regards to permanent implantation, there are 2 methods currently utilized-percutaneous and open “paddle” lead placement. This section of the article will focus on percutaneous lead implant, as open “paddle” lead placement is performed by neurosurgical and orthopedic spine colleagues via laminotomy. The choice between the 2 techniques is dependent on patient needs, local practice patterns, as well as physician training and comfort with surgical skills.
The percutaneous implantation of the stimulation leads as well as internal pulse generator (IPG) occurs in an operating room setting with strict adherence to sterile technique. Of additional concern for implantation is the anesthetic technique utilized. There has been a significant shift over the last decade to performing this procedure under general endotracheal anesthesia with the utilization of intraoperative neuromonitoring. The use of intraoperative neuromonitoring is crucial to prevent and promptly identify potential neural injury.
Should general endotracheal anesthesia with intraoperative neuromonitoring not be utilized, the patient should be awake and conversant throughout with minimal sedation administered.
The initial exposure consists of 2 incisions over the lumbar spine as well as flank. The lumbar incision should then be dissected to the level of the thoracolumbar fascia and the flank incision should be dissected to 2-4 cm below skin in accordance with manufacturer’s recommendations. Meticulous hemostasis should be performed with care taken to not over utilize cautery and potentially damage otherwise healthy tissue. The utilization of epinephrine in local anesthetic infiltration is helpful in this regard. The stimulating leads are then placed in a similar fashion as the trial leads as described above.
The anchoring of the leads to the thoracolumbar fascia should be considered a critical portion of the procedure. Anchoring of the stimulation leads is primarily performed to achieve stability and prevent migration. However, a secondary function of the anchor is to protect the portion of the lead traversing the fascial layer and mitigate the risk of lead fracture. Numerous anchor designs are currently available on the market, and vary between manufacturers. Attention should be paid to proper utilization of the anchoring system to prevent postoperative complications.
After copious irrigation, the lumbar and IPG wounds are then closed in a multilayer fashion using absorbable suture. Multiple layers add redundancy and additional strength to the postoperative wound. Depending on the suture used, tensile strength of the suture can remain for 4-6 weeks allowing for adequate tissue healing. The skin can then be approximated using staples or by utilizing an absorbable suture in a subcuticular manner. After emergence from anesthesia, the patient is taken to the recovery area where initial programming would occur. The patient should be given clear instructions regarding bandaging and activity restrictions prior to discharge.
Postoperative care
Most patients are discharged home the same day as their implant surgery. Patients may experience discomfort at the sites of their incisions after the local anesthetic wears off, but this can be managed in most cases with a 2-3 day course of prescription analgesics. Patients should be advised to avoid sudden movements and minimize bending or twisting for a minimum of 6 weeks postoperatively, to reduce the chance of lead migration or wound dehiscence. An occlusive dressing is maintained over the incision for 24-48 hours postoperatively for primary closures. , The Neurostimulation Appropriateness Consensus Committee recommends antibiotics be discontinued within 24 hours of surgery, though postoperative antibiotics may be considered in high-risk patients. Patients are routinely seen in the office 10-14 days after surgery, though earlier follow-up may be arranged in high-risk patients. Patients are evaluated for appropriate wound healing and observed for any signs of surgical site infection. Nonabsorbable sutures or staples are removed 10-14 days after surgery. Sterile gloves are worn, and the skin overlying the wound prepped with an antiseptic. The skin is palpated to observe for any areas of tenderness or fluctuance. It is common to see a 2-3 mm area of erythema along the incision. Any more than this, or drainage, swelling, or increasing pain should prompt further investigation. Every other suture or staple may be removed initially to ensure good wound integrity. After suture or staple removal, it is our practice to re-dress with steri-strips any patients who may be at increased risk of dehiscence based on body habitus or medical status. Patients are instructed that they may begin showering following staple removal, though there is some evidence from spine surgery literature, that in stapled wounds showering could begin earlier. Patients and caregivers are educated on the signs and symptoms of SSI and are advised to contact the office immediately should these occur. Reassessments in the office are scheduled for 1-month postoperative, and 3-months postoperative. Patients are advised not to submerge bathe until cleared at the 1-month postoperative visit.
Complications
Complications usually present within 3 months of implant surgery. The most common surgical complications include infection (5%), wound hematoma, and seroma. Other surgical complications might include wound dehiscence, dural puncture, epidural hematoma, perforated viscus related to tunneling, spinal cord injury or spinal canal stenosis (6,7). , Device complications include lead migration (24%), lead failure (7%), and pulse generator failure (2%). Patients may also complain of persistent pain in the area of device placement that is unrelated to infection or other processes. For the purposes of this review, we will discuss the more common postoperative issues.
Superficial infection, deep infection, epidural abscess and hematoma
Postoperative laboratory tests are not routinely performed on patients. Pain, swelling, or redness over the surgical site, or fever, chills, or drainage in the postoperative months should be evaluated. If there is concern for SSI, then WBC with differential, ESR, and CRP are performed. CRP may be more predictive of SSI if it does not normalize postoperatively or continues to rise. A normal CRP can help rule out SSI. Any drainage should be cultured, and empiric antibiotics started prior to identification of bacterial growth and sensitivities. Superficial infections near the IPG site may be treated with oral antibiotics, though suspected deeper infections or those near the neuraxial site of entry will likely require device removal and wound irrigation and debridement. , Involvement of infectious disease and wound care specialists may help with complicated wound issues or those involving the neuraxial entry site. The incidence of epidural abscess with implantable devices is unknown. CT or MR imaging may be used to identify the presence of deep pocket or neuraxial infections, or epidural hematoma.
Epidural abscesses will likely require drainage or surgical decompression in addition to prolonged antibiotic treatment. They may present as local tenderness and back ache with later progression to radicular pain and fever, meningeal signs, and paralysis if not promptly treated. Fever and other constitutional symptoms may be present. Mortality from epidural abscess may range from 10%-23%, while permanent neurologic deficit is likely if >12 hours after onset. , Epidural hematomas may have a similar presentation and progression, without the fever, constitutional symptoms, or meningeal signs of epidural abscess. They usually present sooner in the postoperative course than an epidural abscess, 24 hours to 7 days, but should also prompt emergent evaluation and treatment as prolonged neurologic deficits are unlikely to resolve. Spine surgery consultation is recommended the moment epidural hematoma or abscess are in the differential, even prior to confirmatory imaging.
To help reduce the chance of infectious related complications, surgeons should consider incorporating the NACC recommendations for infection prevention and management in all phases of patient care: preoperative evaluation and patient selection, intraoperative procedures, and postoperative care.
Wound dehiscence, hematoma, seroma
Partial or complete separation of the surgical wound may most commonly present during the first 5-8 days after surgery, when the wound is the weakest. It may occur in later phases, though, with poor wound closure such as those under tension near the IPG or other device hardware, in patients with increased medical risk factors, or in patients with inappropriate postoperative movement patterns. Dehiscence is more common in patients with diabetes, uremia, immunosuppression, cancer, obesity, chronic steroid use, or positive smoking status. Wounds that are closed under tension lead to tissue ischemia and poor healing. Tissue layers should be approximated, and appropriate strength sutures should be placed at intervals no wider than 1 cm. Wound dehiscence should be considered the result of infection until proven otherwise. Dehiscence due to infection, or that which exposes the device, will necessitate device removal.
Hematomas or seromas may occur at either the IPG or lead implantation sites. Hemostasis and gentle closure may reduce the chance of wound hematoma. Hematomas may present with swelling and blue to purple skin discoloration, though superficial discoloration is not always present. Because blood is a culture medium, there is some increased risk of infection. Likewise, seromas, which are collections of serum, liquefied fat, and lymphatic fluid, may present as swelling in the surgical area and may be hard to differentiate from hematoma or infection. Any increased erythema or progressive pain in an area of swelling should prompt further evaluation to rule out infection or rapidly expanding hematoma, both of which could lead to wound dehiscence. Additionally, for suspected seroma near the neuraxial entry site, CSF hygroma may also be in the differential. Small hematomas and seromas will often resolve on their own, and surgical exploration may be more risky than observation. Some providers suggest the use of an abdominal binder to decrease the chance of seroma or to help manage after presentation. Any painful or progressive hematomas or seromas will likely need surgical exploration and may result in device removal.
Loss of capture, lead fracture, lead migration
Loss of efficacy due to loss of stimulation capture may result from tolerance to stimulation, lead migration, lead fibrosis, or lead failure. Lead reprogramming is often the first step in trying to overcome loss of stimulation efficacy. Inability to improve therapy with reprogramming should prompt further investigation. Interrogation of lead impedance during reprogramming is helpful as impedance may be increased with lead fibrosis or lead fracture. Increased impedance from suspected lead fracture will need to be addressed as patients with this issue will likely be unable to have MRI until this is corrected. AP and lateral XR of the thoracic spine with comparison to the implant views is used to evaluate lead migration. Careful attention to anchoring technique of percutaneous leads to the dorsal lumbar fascia is important to reduce the likelihood of lead migration. Loss of capture due to any of these processes may result in revision implant surgery, and placement of a paddle lead with a spine surgeon may be considered.
IPG and pocket issues
Several issues may be encountered at the site of battery implant. A pocket that is too large may increase the chance of seroma and device flipping. Care should be taken to ensure appropriate pocket dissection and closure that eliminates dead-space. An anchor suture may be placed in the IPG that will reduce the chance of the device flipping. Device erosion may occur if a pocket dissection or tunneling is too shallow, or in patients with poor tissue health (smoking status, chronic disease, malnutrition). Persistent pain in the area of the IPG may result from mechanical irritation or tissue healing issues (infection, seroma, hematoma). Care should be taken preoperatively to plan IPG placement in an area that will be unlikely to cause irritation from normal patient movement patterns, patient attire (clothes waistline/belts), or other ADLs like sleeping. Persistent pain at the generator site, especially if erythema is present, should elicit investigation before tissue breakdown and may result in device removal.
Conclusion
Spinal Cord Stimulation is an effective method of improving pain control in several chronic disease states. The expanding utilization and indications of this therapy is an opportunity for multidisciplinary teams to improve outcomes. The safe and efficacious usage of this therapy is dependent on technique and adherence to established protocols and guidelines.
Disclosure: The authors report no potential conflicts of interest.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree