15 Hepatocellular Carcinoma: Staging and Clinical Management
K. V. Narayanan Menon, Arvind R. Murali, Baljendra S. Kapoor, and Federico N. Aucejo
15.1 Introduction: Definition and Epidemiology
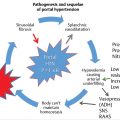
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and is the fifth most common cancer in men and the seventh most common cancer in women worldwide. 1 Men are more commonly affected than women. The male-to-female ratio has been reported to vary regionally from 2:1 to 4:1, 2 with an overall male-to-female ratio of 2.4:1. In the United States, the incidence rates of liver cancer per 100,000 person-years are 2.0 for women and 3.7 for men, with a male-to-female ratio of 2.83:1.
High-incidence areas of HCC include Sub-Saharan Africa and eastern Asia, whereas Canada and the United States are low-incidence areas for HCC; this is because of the increased prevalence of hepatitis B in Sub-Saharan Africa and eastern Asia. The presence of hepatitis B and C increases the risk for cirrhosis, which is seen in 80 to 90% of patients with HCC. However, recent data have shown a downward trend of HCC in eastern Asia, whereas the incidence is increasing in North America. 3
Viral hepatitis is the main risk factor for the development of cirrhosis and HCC, although other risk factors such as alcohol abuse, diabetes mellitus, and obesity can also lead to the development of HCC. Diabetes mellitus can predispose patients to the development of nonalcoholic steatohepatitis, which can subsequently progress to cirrhosis, thus increasing the risk for HCC. It has been reported that obese individuals (body mass index > 30 kg/m2) have higher HCC-related mortality rates than leaner individuals. 4 With the increasing incidence of obesity, the mortality rates associated with HCC could very well increase, as well.
Other etiologies that predispose patients to the development of HCC include hereditary hemochromatosis, alpha-1-antitrypsin deficiency, primary biliary cirrhosis, autoimmune hepatitis, glycogen storage disease, and other hereditary metabolic conditions.
15.2 Surveillance and Staging of Hepatocellular Carcinoma
Surveillance should be performed in patients at high risk for developing liver cancer (▶ Table 15.1). Decision analysis models have demonstrated that surveillance for HCC in patients with cirrhosis is cost effective and improves survival if the incidence of HCC exceeds 1.5% per year. 5 Based on this model, all patients with cirrhosis irrespective of the etiology should be screened biannually. This includes patients with cirrhosis secondary to hepatitis C, hepatitis B, hemochromatosis, primary biliary cirrhosis, primary sclerosing cholangitis, nonalcoholic steatohepatitis, alpha-1-antitrypsin deficiency, and autoimmune hepatitis. Surveillance is also indicated in select hepatitis B carriers (▶ Table 15.1); in these patients, a history of HCC in a first-degree relative is an independent risk factor for the development of HCC. 6 Also, Africans with hepatitis B have been shown to develop HCC early in life. 7 Although initiation of HCC surveillance at a younger age has been recommended for these patients, 8 the exact age at which surveillance should begin has not been clearly established. In addition, it is not clear whether Black patients born outside of Africa are at an increased risk for HCC.
Cirrhosis from any etiology |
Asian male hepatitis B carriers aged > 40 y |
Asian female hepatitis B carriers aged > 50 y |
Hepatitis B carriers with a family history of HCC |
African/North American Black patients with hepatitis B |
Source: Data from Bruix et al. 27 |
HCC surveillance has been shown to decrease mortality. In a randomized controlled trial in China, patients with hepatitis B were either not screened or were screened with abdominal ultrasound and analysis of alpha-fetoprotein (AFP) levels; screening was associated with a 37% decrease in mortality. 9 Studies have also established that patients with early-stage HCC have better survival than those with more advanced disease, 10 , 11 which is largely explained by the availability of effective treatment, including liver transplant, for patients with early-stage cancer. Therefore, asymptomatic patients with early-stage disease who are identified by a surveillance program should have improved survival compared with symptomatic patients.
The most commonly used surveillance methods for HCC are analysis of serum AFP levels and liver ultrasound. Analysis of serum AFP alone has not been shown to be useful, whereas the combination of AFP plus ultrasound has been shown to reduce mortality when used for HCC surveillance. 9 A recent study reported that the combination of AFP and ultrasound had a higher sensitivity (90%) when compared to ultrasound alone (58%) but at the expense of a lower specificity. 12 Studies have also shown that AFP alone has a low sensitivity (54%) for diagnosing HCC. 13 Tumor size has been described as one of the factors limiting the sensitivity of AFP, 13 suggesting that evaluation of AFP might not be useful in the detection of early-stage HCC. Ongoing studies suggest that analysis of AFP-L3, an isoform of AFP, may be helpful in patients with AFP levels in the intermediate range. 14
The current American Association for the Study of Liver Diseases (AASLD) guidelines recommend that ultrasound alone be performed once every 6 months for HCC surveillance. However, it may be premature to conclude that AFP analysis is no longer required for surveillance of HCC, as liver ultrasound is operator dependent, and its efficacy may be negatively affected in overweight or obese individuals.
Patients at high risk for the development of HCC should enter surveillance programs. 8 Patients on the waiting list for a liver transplant are also screened for HCC, as priority, or exclusion from the list is dictated by the extent of HCC as defined by the Milan criteria. 6
Contrast-enhanced multiphase computed tomography (CT) or magnetic resonance imaging (MRI) can be used as an alternative screening modality for individuals in whom ultrasound is suboptimal or nondiagnostic (e.g., patients with extremely fatty or nodular livers).
Any abnormality (e.g., a new or enlarging nodule) detected by screening requires additional investigation and/or follow-up to confirm the presence of HCC as follows (▶ Fig. 15.1):
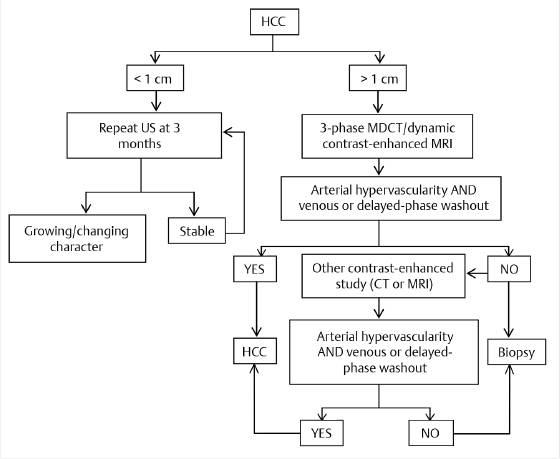
Nodules measuring < 1 cm by ultrasound surveillance should be reassessed with ultrasound in 3 to 6 months. If the nodule is stable for 2 years, routine 6-month surveillance is resumed.
Nodules measuring > 1 cm should be investigated with multiphase contrast-enhanced CT or MRI using the technical specifications outlined by Organ Procurement and Transplantation Network (OPTN)/United Network for Organ Sharing (UNOS; ▶ Table 15.2). 15
Although MRI provides better contrast resolution than CT, MRI is contraindicated in patients with certain metallic implants (e.g., pacemakers) and is more time consuming than CT. MR image quality is degraded by respiratory artifacts, some metallic objects (e.g., surgical clips), and perihepatic ascites, and MRI is not well tolerated by patients with claustrophobia. All of these factors should be taken into consideration when choosing the appropriate imaging modality for the patient.
The risk of nephrogenic systemic fibrosis and radiocontrast-induced nephropathy in individuals undergoing contrast-enhanced MRI and CT, respectively, is a special concern in the subset of patients with severe renal insufficiency (glomerular filtration rate < 30 mL/min/1.73 m2). The relative risks and benefits of using intravenous contrast in this patient population must be considered by the referring physician, the radiologist, and a nephrologist. Contrast-enhanced examinations that are determined to be critical to clinical management take precedence over cautionary measures and can be performed using prophylactic techniques after the radiologist obtains an informed consent from the patient. 16 In some instances, noncontrast MRI of the liver may be of value.
If the imaging appearance is typical of HCC, the lesion should be treated as HCC.
Radiologic criteria typical of HCC include late arterial phase enhancement and delayed phase washout or hypervascularity with short-term growth as outlined by the OPTN classification system for nodules identified in the setting of cirrhosis (▶ Table 15.2). If features are atypical for HCC, a second contrast-enhanced study can be performed or the lesion can be biopsied. If the biopsy is negative, the lesion should be reassessed every 3 to 6 months until the nodule resolves, grows, or exhibits imaging characteristics typical of HCC.
15.2.1 Staging
Multiple staging systems are available for the management of HCC. However, no single staging system has been found to reliably predict outcomes of HCC. This is largely because the prognosis of HCC depends not only on the stage of the cancer but also on the degree of the underlying liver dysfunction. Various staging systems in use take into account the size of the tumors, severity of underlying liver disease, extension of the tumor into adjacent structures, and presence or absence of metastases. The most common staging systems used are the TNM staging system, the Okuda staging system, the Barcelona Clinic Liver Cancer (BCLC) system, the Chinese University Prognostic Index (CUPI), the Cancer of the Liver Italian Program (CLIP) score, and the biomarker-combined Japanese Integrated Staging (JIS) system (▶ Table 15.3). The BCLC has been externally validated in Western populations, whereas the JIS has been externally validated in Eastern populations. Because of regional differences in the risk factors for HCC and other comorbidities, a common global classification system has not yet been established. 17
The TNM staging system is commonly used for all solid malignancies. It involves assessment of the primary tumor size (T), regional lymph node involvement (N), and metastases beyond the regional lymph nodes (M). TNM staging for HCC is performed clinically by means of imaging studies, but the presence of a cirrhotic nodular liver and benign enlargement of lymph nodes that are commonly seen in patients with advanced cirrhosis make clinical TNM staging in these patients challenging. Pathological TNM staging is performed in patients who have undergone resection of HCC. Pathological TNM staging has been shown to be superior to the Okuda, CLIP, and CUPI systems among patients who have undergone curative resection of HCC. 19 The main drawback of pathological TNM staging is that it can be used in only a minority of patients, as very few patients with HCC undergo surgical resection. In addition, TNM staging does not take into account the severity of liver dysfunction and hence may not be accurate in patients with advanced cirrhosis.
The Okuda staging system is based on both tumor characteristics and the degree of liver dysfunction. This classification involves four factors: tumor size (involving > 50 or < 50% of the liver), serum albumin level, serum bilirubin level, and presence or absence of ascites. Patients with tumor involving < 50% of the liver, albumin level > 3 g/dL, bilirubin level < 3 mg/dL, and no ascites are considered to have Okuda stage I disease; these patients have higher survival rates than those with Okuda stage 2 (one to two factors) or stage 3 (three to four factors) disease. 20 The major drawback of this classification system is that a number of cases are identified as Okuda stage 1 through HCC screening, and this system lacks discrimination among Okuda stage 1 cases.
The BCLC staging system for HCC is the most commonly used staging system. It is endorsed by the European Association for the Study of the Liver (EASL) and the AASLD. The BCLC system incorporates tumor characteristics (tumor size, tumor extent, portal venous invasion, and presence or absence of metastasis), severity of liver dysfunction (Child–Pugh score, portal hypertension), performance status of the individual, and constitutional symptoms from HCC into the disease score. 21 Based on these factors, cases are divided into four categories (BCLC class A, early disease; class B, intermediate disease; class C, advanced disease; and class D, end-stage disease). Treatment recommendations have been provided for each BCLC class. The BCLC classification system has been externally validated in Western populations and has been identified as the best independent prognostic predictive indicator versus six other staging systems in the United States. 22
The CLIP system uses four factors (Child–Pugh score, tumor morphology, AFP level, and presence/absence of portal vein thrombosis) to determine HCC disease stage. 23 Each factor is assigned a score of 0, 1, or 2, with the total score ranging from 0 to 6. This scoring system has been validated prospectively in an Italian cohort, but in the Japanese population, the median survival for each CLIP score was higher than that in the Western population. 17 , 24 In addition, the group with the best prognosis in the CLIP staging system is considered to have advanced HCC; therefore, the ability of this system to identify patients who would benefit from curative therapy has been questioned. 25
The JIS system was developed and proposed by the Liver Cancer Study Group of Japan. 25 This system incorporates tumor characteristics (Japanese TNM staging system) and the degree of liver dysfunction (Child–Pugh class). The JIS system has been validated in the Japanese population, with excellent discrimination demonstrated among patients with early-stage HCC. The JIS system has been further modified by adding biomarkers (AFP, lecithin reactive AFP, and des-gamma-carboxy prothrombin) to the model, and this modified system was shown to have a better prognostic value than the original JIS model. 26 However, the JIS and modified JIS systems have not been validated in Western populations.
The multitude of factors affecting prognosis in patients with HCC has made it challenging to develop a universally accepted staging system. In addition to tumor morphological characteristics and the severity of liver dysfunction, tumor biology and the etiology of liver disease (such as nonalcoholic fatty liver disease) may influence prognosis. Further research is clearly needed to better define staging systems for HCC and to determine appropriate treatment strategies for each stage of HCC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree