1. Massive
> 300 mL/24 h
2. Moderate
> 3 episodes of > 100 mL/day in one week
3. Mild
< 100 mL/day × 3 per week
Table 6.2
Another classification uses the terms “major or significant” and mild
1. Major or significant | > 240 mL/24 h > 100 mL/day, recurrent < 100 mL/day, but chronic or recurrent + Interferes with lifestyle Prevents effective physiotherapy Prevents home management |
2. Mild | Does not fit above criteria |
Most authors agree that massive hemoptysis requires intervention due to the risk of death. However, consensus is not present on the treatment of chronic or recurrent hemoptysis. Chronic or recurrent hemoptysis is debilitating, interferes with chest physiotherapy and aerosol treatment, and is related to an increased number of exacerbations and lung destruction. Chronic recurrent bleeding is more common in patients with severe lung disease (FEV1 < 50 %) [14, 15].
Initial therapy in patients with hemoptysis is medical. CF patients are typically admitted, chest physiotherapy is discontinued along with penicillins and nonsteroidal anti-inflammatory drugs, and patients receive vitamin K and antibiotics [1, 4, 8, 9]. Blood transfusions may be given if indicated. Holsclaw et al. described 19 patients with massive hemoptysis that received medical management [16]. There was 32 % mortality, and 50 % of the survivors had repetitive hemoptysis with total mortality over 6 months of 68.4 %. A somewhat later study by Stern showed 100 % survival with medical management in the setting of massive hemoptysis, with a 45 % rebleed rate [17]. Tranexamic acid is a fibrinolysis inhibitor that has been used for medical management [1, 4, 18]. The typical dose for massive hemoptysis is 15–25 mg/kg IV every 6 h for four doses. For less severe hemoptysis, it can be given orally at a dose of 1 g three times a day for 5–6 days [1, 8]. Vasopressin and octreotide (a selective bronchial artery vasoconstrictor) have also been tried in the past [1, 8]. A recent report described the use of factor VII in treating refractory hemoptysis [13].
Endoscopic treatments include cautery or laser, balloon occlusion of the bronchus, topical drugs such as epinephrine, and selective intubation [1, 3, 6]. Surgical options include lobectomy and extrapleural bronchial artery ligation [1, 3, 11]. However, angiography and embolization have mostly replaced these options as a first-line treatment of hemoptysis [2, 3, 11, 13].
Arterial supply to the lung is primarily from the pulmonary arteries (99 %) with the bronchial arteries supplying the remainder of the flow (1 %) predominately to the mediastinum, airways, lymph nodes, and visceral pleura [5]. While the pulmonary arteries have a lower pressure, bronchial arteries have systemic blood pressure. These bronchial arteries are the cause of hemoptysis in 90 % of patients with CF and are the most common source of hemoptysis in patients with other inflammatory conditions and tumors due to their hypertrophy and angiogenesis [2, 5].
Bronchial artery anatomy is variable, but knowledge of these arteries and their possible communications is critical in treating patients with hemoptysis [1, 3]. A postmortem study showed 80 % of bronchial arteries arise from the aorta at the 6th–7th thoracic vertebral level (see Table 6.3 for distribution) [19]. Table 6.4 shows the distribution from an angiographic study by Uflacker et al. [20] (Fig. 6.2).
Table 6.3
A postmortem study showed 80 % of cases arise from the aorta at the 6th–7th thoracic vertebral level and had the following distribution [19]
41 % | One right | Two left |
21 % | One common | |
21 % | Two right | Two left |
10 % | One right | One left |
31 % | One right intercostal bronchial trunk (RICBT) | One left |
25 % | One RICBT | One common trunk |
13 % | One RICBT | Two left |
11 % | One RICBT | One right, one left |
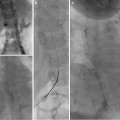
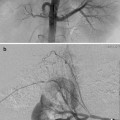
The origin of the RICBT is typically posterolateral, the right bronchial artery is lateral to anterior, the common trunk arises anteriorly, and the left bronchial artery may be anterior, anterolateral, or from the aortic arch. The RICBT supplies the right lung in 80 % of patients. It occasionally supplies the left lung and the superior intercostal artery and provides supply to the spinal artery in 5 % of patients (Fig. 6.1a–c) [5]. In addition, the RICBT may have collaterals to the subclavian artery, the mid-esophagus, the pericardium, and the coronary arteries [20].
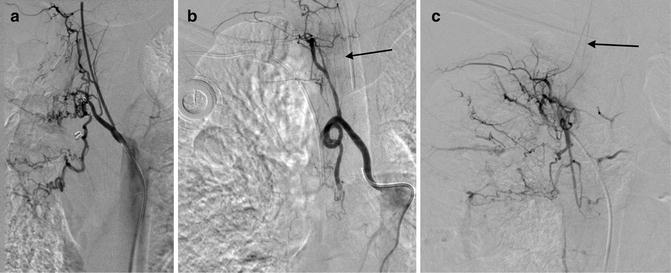
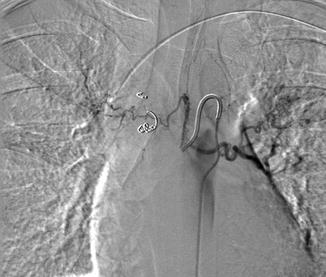

Fig. 6.1
These are three examples of the right intercostal bronchial trunk. In the last two images, anterior medullary radicular arteries are identified feeding spinal artery branches (arrows)

Fig. 6.2
Common bronchial artery trunk
In addition to these variations, there are aberrant origins of the bronchial arteries including the subclavian arteries and their branches (internal mammary artery (IMA), thyrocervical artery, costocervical artery, lateral thoracic artery), the superior intercostal artery, the inferior phrenic artery, the left gastric artery, and esophageal artery branches (Figs. 6.3a–e) [5, 20]. There are often recruited transpleural collaterals from the subclavian artery and its branches as well that can be identified by their supply to the pleura and subsequent filling of intrapulmonary branches (Fig. 6.4) [1, 2, 5].
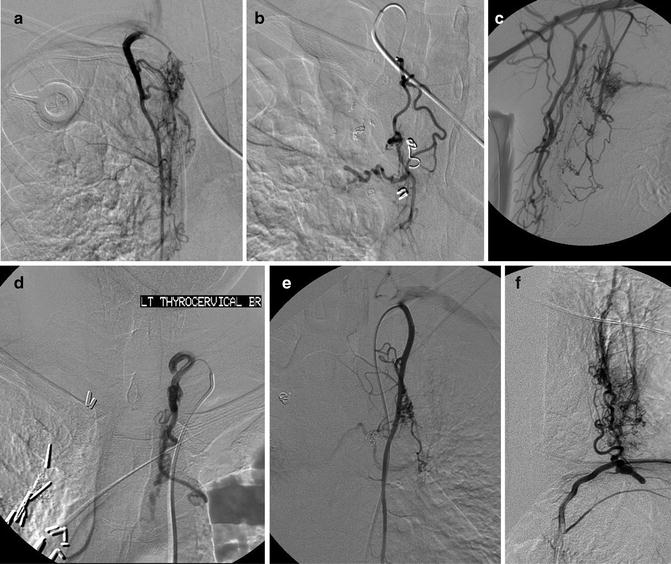
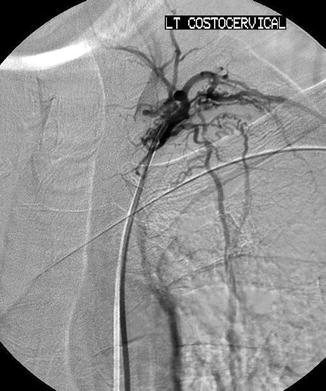

Fig. 6.3
Multiple aberrant origins of the bronchial arteries including the right internal mammary artery (a), right thyrocervical artery (b), right lateral thoracic artery (c), left thyrocervical artery (d), left internal mammary artery (e), and left phrenic artery (f)

Fig. 6.4
This is a transpleural collateral from the left subclavian artery. Noted is filling of the pulmonary veins
As stated above, the RICBT may provide supply to the anterior spinal artery, and other branches may supply the anterior spinal artery as well via anterior segmental medullary arteries. These are identified by a classic hairpin turn and the largest of these is named the artery of Adamkiewicz which usually originates at the T9–12 level [1, 5, 6]. Identification of these arteries is crucial during the embolization procedure, as inadvertent embolization can cause spinal cord ischemia [6, 11].
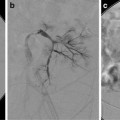
There are many connections between the bronchial arteries, aberrant bronchial arteries, and the transpleural collaterals; therefore, any artery may potentially supply the spinal artery or connect with the subclavian artery and its branches (Fig. 6.5) [21]. One third of arteries responsible for hemoptysis are non-bronchial systemic collaterals [3, 7, 11].
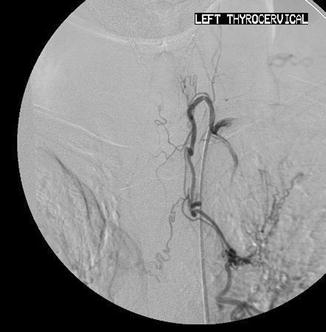

Fig. 6.5
Injection of the left thyrocervical artery demonstrates collateralization with right and left bronchial arteries and multiple mediastinal branches
Bronchial artery embolization was first described by Remy in 1974 [22]. The use of this technique for treating hemoptysis has been described in many studies [1–8, 11, 12, 17, 23–26]. The goal of the procedure is to reduce the pressure to the site of hemorrhage allowing the artery to heal while avoiding tissue destruction [1]. A key goal is to allow a pathway for retreatment if necessary as these patients typically have an ongoing disease process, and recurrent hemoptysis is common.
While there have been no controlled studies comparing embolic agents, reported materials include gelatin sponge, polyvinyl alcohol (PVA) particles, spherical embolics, coils, and n-butyl cyanoacrylate (n-BCNA) [1–5, 7, 9, 11, 12, 23, 27]. Gelatin sponge can be quite effective but is a temporary agent [1, 9]. PVA is a permanent agent, although the occlusion is not permanent [1, 2]. The size of the embolic material is more uniform than with gelatin sponge. Shunts to the pulmonary circulation as large as 325 μm have been described, and therefore, the particles used are usually greater than 350 μm in size [5, 27]. Spherical agents have been used, but they need to be sized larger than PVA as they compress and can more easily pass through shunts to the pulmonary veins or arteries [1, 12]. N-BCNA has been described in some studies and is permanent, but the delivery may be more difficult [1]. It may have a lower rate of rebleeding. Coils are contraindicated for proximal occlusion but may be useful for distal arteries in a patient with recurrent hemoptysis or in excluding a nontarget artery [1, 6, 7]. Prior reports using gelatin sponge powder and ethanol have had unacceptable incidences of bronchial necrosis and other complications [1, 6, 8].
Indications/Contraindications
Indications for treatment include those patients with massive or moderate hemoptysis when medical management has failed. Embolization of chronic and recurrent hemoptysis may be indicated when [7]:
1.
They have had mild hemoptysis leading to massive hemoptysis before.
2.
They have hemoptysis within 2 weeks after completing recent optimal medical management.
3.
Hemoptysis continues despite a trial of optimal medical management.
4.
Embolization is performed to stop hemoptysis as a bridge to transplantation.
As described above, there are multiple other causes of hemoptysis in addition to CF (Figs. 6.6 and 6.7).
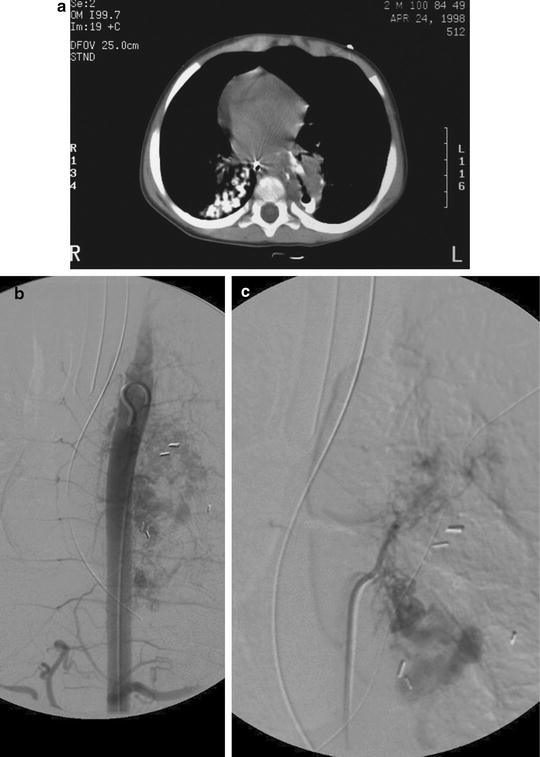
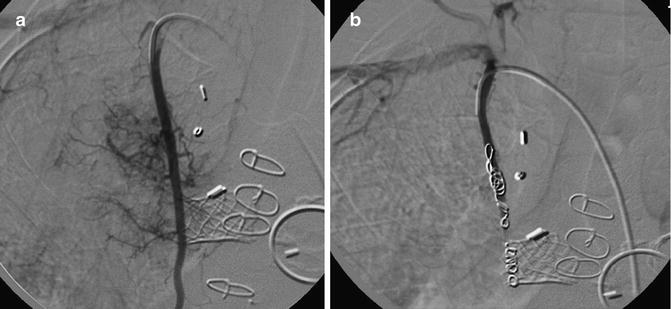

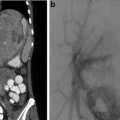
Fig. 6.6
This patient had hemoptysis after resection of the superior segment of the left lower lobe. A CT scan shows stump dehiscence with a contained leak (a). Subsequent aortogram (b) shows an extensive vascular blush in the left paraspinal region with resolution after embolization (c)

Fig. 6.7
Three-year-old patient with recurrent hemoptysis after surgery for congenital heart disease. In (a), an injection of the right internal mammary artery shows innumerable collaterals to the lungs. PVA and coil embolization excluded this artery (b), and with the embolization of multiple other pulmonary artery collaterals, the hemoptysis ceased
Consultation with the patient, family, and referring physicians is imperative including potential risks such as hemorrhage, anaphylaxis, vascular injury, stroke, and spinal cord injury, and possible alternative therapies should be discussed. Standard contraindications to angiography are valid in this setting [28].
Pre-procedural Evaluation
Prior to performing bronchial arteriography for potential embolization, attempts to localize the site of bleeding have been performed. The patient may indicate where they feel a “gurgling” or other sensation. Plain radiographs may show new areas of airspace disease representing hemorrhage (useful in 17–80 % of patients), and a CT scan may be more sensitive and can identify the bronchial arteries [1, 5, 6, 29, 30]. Bronchoscopy may identify the site or side of bleeding in 10–91 % of patients; however, it may fail as blood may be present throughout the bronchi due to coughing [5]. The site cannot be identified in 20–30 % of patients [2].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree