Neurology
Active head bleeding?
( ) Yes
( ) No
Intracranial tumor? Spinal tumor?
( ) Yes
( ) No
Intracranial lesion (AVM, aneurysm)?
( ) Yes
( ) No
Seizure disorder?
( ) Yes
( ) No
TIA? Moyamoya?
( ) Yes
( ) No
Lumbar puncture <7 days?
( ) Yes
( ) No
Recent neurosurgery?
( ) Yes
( ) No
Head trauma or intracranial bleeding <2 weeks?
( ) Yes
( ) No
( ) Yes
( ) No
Hematology
Severe thrombocytopenia (<100,000)?
( ) Yes
( ) No
DIC? Bleeding disorder?
( ) Yes
( ) No
Contraindication to anticoagulation?
( ) Yes
( ) No
( ) Yes
( ) No
Acute events
Active internal bleeding?
( ) Yes
( ) No
Recent GI or GU bleeding (<2 weeks)?
( ) Yes
( ) No
Recent CPR?
( ) Yes
( ) No
Massive PE with hemodynamic compromise?
( ) Yes
( ) No
Major surgery or trauma (last 10 days)
( ) Yes
( ) No
Recent biopsy?
( ) Yes
( ) No
( ) Yes
( ) No
Other systems
Right to left cardiac or pulmonary shunt? HHT?
( ) Yes
( ) No
Bacterial endocarditis or pericarditis?
( ) Yes
( ) No
Myocardial infarction?
( ) Yes
( ) No
Renal failure?
( ) Yes
( ) No
Uncontrolled hypertension?
( ) Yes
( ) No
Hepatic failure?
( ) Yes
( ) No
Recent eye surgery? Diabetic retinopathy?
( ) Yes
( ) No
Allergies: contrast, tPA?
( ) Yes
( ) No
Pregnancy?
( ) Yes
( ) No
Lab values
Platelets <100,000/μL
( ) Yes
( ) No
INR >1.6
( ) Yes
( ) No
PTT prolonged by >4 s
( ) Yes
( ) No
Fibrinogen <100 g/dL
( ) Yes
( ) No
Preprocedure Work-Up
Imaging
Existing imaging is reviewed and further studies ordered if necessary. Thrombosis is most commonly assessed using duplex ultrasound with compression. CT or MR can be utilized for deeper structures or prior to undertaking more complex procedures such as venous recanalization. CTA is performed to assess for suspected pulmonary embolism.
Additional imaging may be required when thrombosis is related to an underlying disease. For example, a patient with DVT and a testicular tumor should undergo brain MRI (or CT) to exclude cerebral metastases prior to considering thrombolysis.
Bloodwork
Complete blood count (CBC), partial thromboplastin time (PTT), prothrombin time (PT)/international normalized ratio (INR), fibrinogen, d-dimers, blood urea nitrogen (BUN), creatinine, and blood group for potential crossmatch should be obtained.
Thrombophilia Testing
Thrombophilia testing is not routinely performed at the time of presentation. Most thrombophilic abnormalities will not have a direct impact on immediate clinical management, and acute derangements in the coagulation system can result in false-positive results. Exceptions to this approach include suspected severe deficiencies of specific coagulation inhibitors (i.e., antithrombin or protein C/S) or an inherited/acquired condition that may require longer anticoagulation due to higher risk of thrombosis recurrence (i.e., lupus anticoagulant) [26, 27].
Testing should be performed in children older than 12–18 months of age, preferably at least 4–6 weeks after anticoagulation has been discontinued. The assessment includes determination of protein S, protein C, and antithrombin deficiencies, factor V Leiden and prothrombin mutations, lupus and anticardiolipin antibodies, and elevations of factor VIII and lipoprotein (a). Thrombophilia screening likely has the greatest benefit in adolescents with spontaneous thrombosis. Thrombophilia testing in asymptomatic patients with a known family history is not indicated [28].
History and Physical
Obtain a thorough description of the acute event and timing; review risk factors for thrombosis and those associated with relative and absolute contraindications (see Table 15.1). Physical examination should include assessment of perfusion, neurologic status/muscle function assessment, leg circumference (for DVT), and pulses/perfusion (where applicable).
Multidisciplinary Discussion
The best treatment approach should be determined through interdisciplinary discussion between thrombosis, interventional radiology, intensive care and, when appropriate, vascular/plastic surgery services.
Commonly Used Drugs and Blood Products
Anticoagulation Drugs
Anticoagulants act by inhibiting the formation of new thrombus. The commonly encountered traditional agents, unfractionated heparin (UH) and warfarin, are now used alongside other anticoagulants such as low-molecular-weight heparin (LMWH) and various forms of antiplatelet agents (i.e., aspirin, clopidogrel, dipyridamole).
Heparin
Heparin is a polymeric glycosaminoglycan that inhibits thrombin, an enzyme necessary for thrombus formation. Due to its short half-life, ease of titration, and the ability to fully reverse its effect with protamine, UH tends to be used for acute thrombotic events, particularly in instances when the perceived risk of bleeding is elevated. To obtain therapeutic heparinization, the standard approach is to give a bolus injection of 75–100 U/kg followed by an infusion of 20 U/kg/h (28 U/kg/h if under 12 months) [29]. Several different laboratory-monitoring modalities are used in clinical practice. PTT (and sometimes activated clotting time [ACT]) and the anti-Xa assay are used to titrate the dose to the appropriate UH levels.
The use of heparinization during thrombolysis is controversial due to a possible increase in bleeding risk [5, 30]. Approaches vary from withholding heparin to administering partial (10 U/kg/h) to full heparinization.
Following intervention, the thrombosis service may commence the use of LMWH with dosing based on age-appropriate regimens and monitoring for therapeutic anti-Xa levels.
Warfarin
Warfarin is a vitamin K antagonist that suppresses synthesis of multiple vitamin K-dependent clotting factors produced by the liver (oral vitamin K antagonist [OVKA]). The complete anticoagulant effects of warfarin take several days, and vitamin K is the most commonly used reversal agent available (given either orally or intravenously, more rapid reversal with the latter). Effectiveness is monitored by assessing the international normalized ratio (INR). When an urgent reversal is necessary, factors can be supplied through administration of FFP, prothrombin complex concentrates (PCCs), or the recombinant activated factor VII (rFVIIa).
Antiplatelet Agents
The formation of a thrombus (blood clot) is divided in two phases. The initial one, in a process called primary hemostasis, is responsible for the formation of a platelet plug. Subsequently, with the participation of the coagulation factors following the coagulation cascade, a insoluble net of protein (ie. fibrin) anchoring the clot to the wound site ensues. Platelet plugs constitute a major player in the formation of thrombi, particularly in damaged vessels with high shear stress of the arterial system. Platelet plugs are formed in the following manner:
(a)
Platelet adhesion—platelet-vessel wall inter-action
(b)
Activation—release of platelet’s internal contents from their alpha and/or dense granules with amplification of the platelet-mediated prothrombotic effects
(c)
Platelet aggregation—platelet-platelet inter-action
Antiplatelet agents are used to target specific molecular interactions preventing thrombus formation. They are used for prophylaxis (endovascular stents, Kawasaki disease) or to prevent thrombus growth (myocardial infarction or ATE).
The most common agents used in children are aspirin, clopidogrel (i.e., Plavix®), dipyridamole, and, rarely, abciximab [31].
Aspirin
Aspirin acts by irreversibly acetylating one of the enzymes (cyclooxygenase-1 [COX-1]) responsible for the generation of one of the most potent platelet agonists, named thromboxane A2. Its inhibition lasts for the entire platelet lifespan. In general, prevention of periprocedural bleeds in children receiving aspirin involves its discontinuation for at least 7–10 days prior to the procedures. The most common side effects are excessive bruising, gastrointestinal toxicity, and the dose-dependent Reye syndrome.
Clopidogrel
Clopidogrel (Plavix®) is part of the thienopyridine family, inhibitors of the platelet agonist, ADP receptor P2Y12. In general, prevention of periprocedural bleeds in patients taking Plavix involves its discontinuation for at least 7 days prior to the procedure. The most common side effects are skin rash, neutropenia, nausea and vomiting, and, rarely, thrombotic thrombocytopenic purpura.
Dipyridamole
Dipyridamole (Persantine®) is a compound commonly used in the setting of clotting prophylaxis in patients post solid organ transplant or in recipients of ventricular assistant devices. It has several mechanisms of action, including changes of adenosine uptake and potentiation of nitric oxide effect. It should also be stopped 7–10 days prior to surgery, and its most common side effects are nausea, flushing, and bleeding.
Abciximab
Abciximab (i.e., ReoPro®) is a monoclonal antibody against the most important platelet agonist relevant for platelet aggregation, glycoprotein IIb/IIIa (GPIIb/IIIa). It is one of three FDA-approved GPIIb/IIIa antagonists resulting in immediate inhibition of platelet aggregation [32]. It is usually utilized as a rescue medical therapy before adult patients are submitted to revascularization or in children, mostly with coronary artery problems (i.e., aneurysms secondary to Kawasaki disease). The major potential adverse events are bleeding and severe thrombocytopenia.
Thrombolytic Drugs
Thrombolytic agents actively break down thrombus. They act by promoting the conversion of plasminogen to plasmin that in turn breaks down fibrin. Plasminogen is found in circulating plasma and bound to fibrin within thrombus. Fibrin-specific thrombolytic agents target bound plasminogen where non-fibrin-specific agents activate both bound and free forms. The activation of free plasminogen acts to deplete plasma proteins and increases bleeding risk. Reported pediatric and adult doses are discussed in Table 15.2.
Table 15.2
Commonly used drugs in thrombosis treatment
Heparin |
Unfractionated |
Initial loading dose: a) anticoagulation alone: 50–100 U/kg/dose; b) Before preprocedures: 100–150 U/kg/dose |
Infusion for “full” heparinization: 20 U/kg/h (28 U/kg/h if less than 12 months olda) |
Partial heparinization: 10 U/kg/h |
TPA a |
Adult (>10 yearsb) |
Arterial |
Bolus: 4–5 mg, can repeat × 2 |
Infusion: 0.5–1 mg/h (at 0.5–1.0 mg/mL concentration) |
DVT |
Bolus: 4–10 mgc |
Infusion: 1 mg in 100 mL NS per hourd |
– Can increase to 2 mg/h if needed |
– For bilateral lower extremity DVT, can run two catheters with 1 mg/h |
Pediatric e |
Systemic |
Low dose: 0.01–0.06 mg/kg/h [3] |
Catheter directed |
Low dose |
– Start at 0.06 mg/kg/h in neonates |
– 0.03 mg/kg/h in non-neonates [3] |
Arterial (non-neonates) |
Bolus |
– 0.1–0.2 mg/kg (5 mg maximum), can repeat × 1 |
Infusion |
– Start at 0.03–0.1 mg/kg/h |
– If not effective, can incrementally increase to systemic dose (0.5 mg/kg/h) |
DVT |
Bolus |
– 0.1–0.2 mg/kg (5 mg maximum), can repeat × 1 |
Infusion |
– 0.03–0.06 mg/kg/h; run at maintenance volume |
Compared to systemic administration, catheter-directed thrombolysis decreases the thrombolytics dose with a potential decrease in associated complications. Administration can be performed on a continuous or intermittent basis [33].
Tissue plasminogen activator (TPA) and urokinase are the two most widely used thrombolytics. Their efficacy and safety is similar in DVT treatment in adults [34].
TPA
TPA is a serine protease that is produced using recombinant DNA technology. TPA has high fibrin specificity with a free plasma half-life of 3–6 min and a thrombin-bound half-life of 2 h.
In vitro studies demonstrate a bell-shaped response curve to TPA concentration with the best results found using q 30 s pulse spray application of 0.01 mg/mL in rabbit thrombus [35]. TPA was originally used clinically in concentrations of 0.5–1 mg/mL. This concentration was based on solubility when TPA is dissolved in balanced salt solution. The same is not true for other types of dilutants including normal saline [36]. Lower concentrations (0.01 mg/mL) are routinely employed for treatment of DVT.
Urokinase
Urokinase (UK) is a direct plasminogen activator that is produced in the urothelium. The half-life is approximately 15 min. UK has a higher affinity for fibrin-bound plasminogen than free plasma plasminogen. The product is created using cell culture techniques. UK was temporarily removed from the market in the USA due to concerns over inadequate microbiologic testing. As a result, urokinase is not in common use today.
Blood Products
Blood products such as packed red blood cells, FFP, or cryoprecipitate may be required to correct derangements during thrombolysis.
Equipment
Thrombolysis Equipment
Standard end-hole catheters can be used; however, specialized 3–5 Fr multi-side-hole infusion catheters and wires (0.035″) come in a variety of infusion lengths. Infusion catheters without a one-way end valve require a tip-occluding wire to assure preferential flow through the side holes. Pulse spray thrombolytic administration can also be performed through infusion catheters. Wires and/or catheters are used alone or in combination to allow the interventionalist to customize the infusion length based on thrombus extent.
A recent innovation, catheter-directed thrombolysis assisted by US thrombus fragmentation (EKOS EndoWave, EKOS, Bothell, WA), may potentially increase the interaction of the thrombolytic agent with the thrombus and reduce the length of thrombolytic infusion [44].
Thrombectomy Equipment
Thrombectomy devices range from simple balloons to specialized catheters and complex machines.
Balloons
Balloons were traditionally used to perform surgical thrombectomy. Fogarty balloons were inserted through a venotomy or arteriotomy and thrombus was extracted. Over-the-wire Fogarty balloons are now available. Traditional angioplasty balloons are used to fragment thrombus or treat underlying stenosis. Balloon fragmentation has been used to treat life-threatening central DVT [40]. Specialized drug delivery balloons have also been used for thrombolytic administration.
Catheters
By applying manual suction with a syringe, any large-bore catheter can be used to perform a thrombectomy. Specially designed manual suction thrombectomy catheters are available.
Specialized Thrombectomy Devices
Numerous, specialized devices have been created to aspirate, macerate, or fragment thrombus. A few of the commonly used examples include AngioJet®, Trellis®, and Arrow-Trerotola PTD®.
Aspiration devices create a vacuum directly or use high-pressure saline resulting in the creation of suction through the Bernoulli effect. The AngioJet® (MEDRAD, USA) is a rheolytic device where internal saline flow creates suction. Four to 6 Fr AngioJet catheters allow treatment of vessels ranging in size from coronary arteries to large veins involved in DVT.
Another device that is in widespread use today is a pharmacomechanical isolation thrombectomy catheter Trellis® (Covidien, USA) available in 6 and 8 Fr sizes. The device uses two balloons to isolate a segment of thrombosed vessel. Thrombolytic agent is then injected between the balloons and is agitated with a sine wave generator by the intervening wire segment. The thrombus fragments are then aspirated prior to deflating the balloons to avoid the risk of distal embolization (Fig. 15.1).
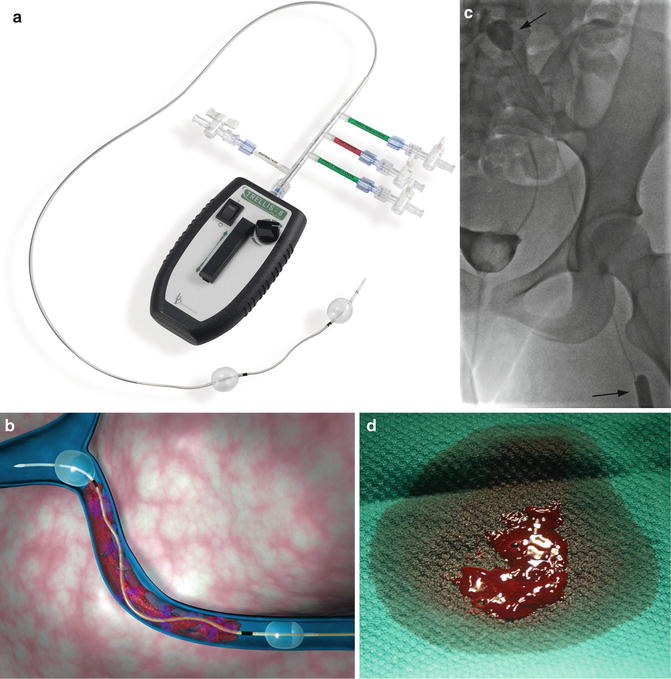

Fig. 15.1
TrellisTM peripheral infusion system. (a) Overview of device demonstrates balloons used to isolate a segment of thrombus. Thrombolytic is sequestered between the balloons, agitated with the motor-driven wire, and aspirated. (b) Computer-generated representation of device in use. (c) Fifteen-year-old female with iliofemoral DVT undergoing treatment with TrellisTM. The balloons (arrows) isolate the thrombus during treatment. (d) Image of thrombus fragment obtained after aspiration. With permission © Covidien AG, 2013
The Arrow-Trerotola PTD® (Teleflex, USA) is an example of a mechanical thrombectomy device that is FDA approved for arterial and venous thrombectomy. It has primarily been used to treat hemodialysis grafts. This device a motor-driven fragmentation device rotating when deployed at 3,000 rpm. The fragmented thrombus can then be evacuated through the compatible 7 Fr introducer sheath or larger sheath [45].
Other approaches such as ultrasound wires [46] and high-intensity focused ultrasound/histotripsy [47, 48] have been described but are not in widespread use at this time.
Some thrombectomy devices can cause fragmentation of thrombus resulting in pulmonary embolism or hemolysis leading to hemoglobinuria and the possibility of renal impairment.
Chronic Thrombosis
Treatment of chronic thrombosis can be accomplished and have benefit even years after the index thrombotic event [49, 50]. Recanalization can be accomplished using a sharp needle (see Fig. 9.16, Chap. 9) or specialized equipment such as a radiofrequency wire (Fig. 15.2) [51, 52].
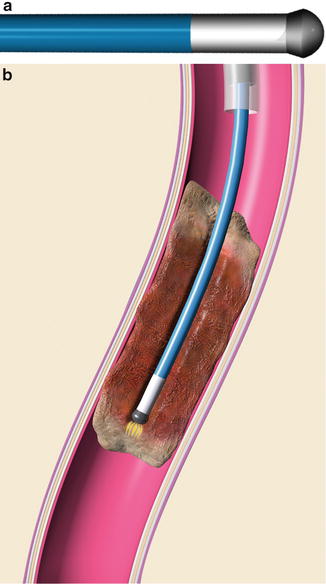

Fig. 15.2
(a) Graphic representation of RF tip of wire. (b) Radiofrequency energy is used to create a channel through fibrotic regions of chronic thrombus. With permission. © Baylis Medical Company Inc. 2013
Procedure Technique
Below is a basic outline of endovascular thrombosis management. The exact steps are dictated by the specific approach being utilized. More detailed information for venous and arterial thrombosis intervention is included in the sections below.
Procedure Preparation
Informed consent is obtained. For younger patients, assent should be obtained where appropriate. An ICU bed is booked if thrombolysis might continue after procedure. When using a thrombectomy device that can cause hemolysis, administration of (isotonic) double-maintenance fluid should be considered to reduce the risk of renal tubular toxicity. Pharmacy should be contacted to allow adequate preparation time and timely delivery of lytic agent.
An arterial line can be placed to allow invasive blood pressure monitoring and easy collection of blood samples. Thrombolysis patients who will require ICU admission and ongoing bloodwork should undergo insertion prior to infusion of TPA to minimize potential bleeding complications. A large-bore IV is placed for administration of fluids and blood products.
Sedation
Sedation/anesthesia is based on the needs of the patient, procedure, and institutional availability/practice. See Chap. 3.
Positioning
Arterial interventions are performed with the patient in supine position. Positioning for DVT intervention is determined by the access route. The patient is placed in prone position when accessing the popliteal vein and supine position for the femoral vein, posterior tibial vein (or other patent calf vein), or upper extremity access. If an IVC filter is being placed, the patient may need to be repositioned during the procedure. Some operators avoid this issue by inserting the filter through a popliteal approach with the patient in prone position [53].
Filter Insertion
Placement of IVC filters during thrombosis treatment is controversial. Insertion of IVC filters has been demonstrated to decrease the incidence of pulmonary embolism in patients undergoing endovascular VTE treatment [53]. When used, venous filters are placed at the beginning of the procedure. In very small patients filters may not open fully resulting in concerns related to the overall length and efficacy. Chaudry et al. described successful, uncomplicated IVC filter insertion performed in three patients with an IVC diameter less than 1 cm [54]. Filter insertion techniques are described in Chap. 11.
Vascular Access
The location of thrombus, the type of vessel involved (artery vs. vein), the vessel size, and the potential for causing further (iatrogenic) thrombosis affect the choice of access site and route.
Whenever possible, antegrade access below the level of thrombosis is utilized for DVT treatment. This minimizes the potential for creating further valvular damage and improving efficacy by allowing establishment of venous inflow and outflow.
Transhepatic access is used for portal vein interventions (Fig. 15.3). Alternatively, antegrade access through the superior mesenteric vein can be obtained during laparoscopy if a combined surgical-radiological approach is desired.
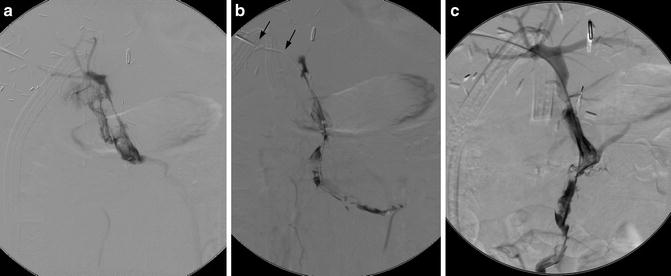

Fig. 15.3
Portal vein thrombosis following liver transplantation. (a, b) A transhepatic catheter (arrows) demonstrates portal and superior mesenteric vein thrombosis. (c) Following pulse spray thrombolysis, thrombectomy, and 48 h of TPA infusion, portal vein flow has been restored. Thrombus in the splenic vein (not shown) completely resolved, thus providing adequate flow to maintain portal vein patency
As there are no valves within the arterial system, an antegrade or retrograde approach can be used.
Insertion of a vascular sheath will minimize the potential for vessel damage related to catheter changes and provides an infusion port for administration of drugs and contrast. The size of vascular sheath is based on the device and/or balloon used (3–9 Fr).
Define Thrombus Extent
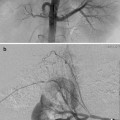
DSA venography/angiography is used to define the extent of thrombosis and the presence of collateral pathways (Figs. 15.4a–c and 15.5a, b). This information helps determine the best procedural method. For example, escape of lytic agent through large collaterals around an area of DVT may make use of the Trellis less desirable than other devices.
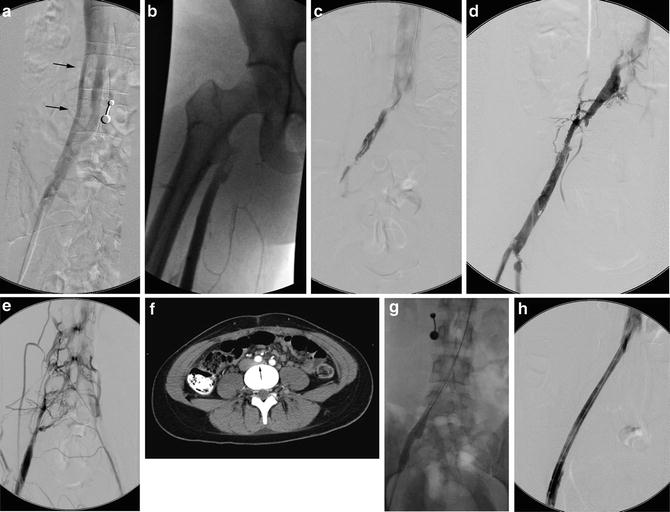
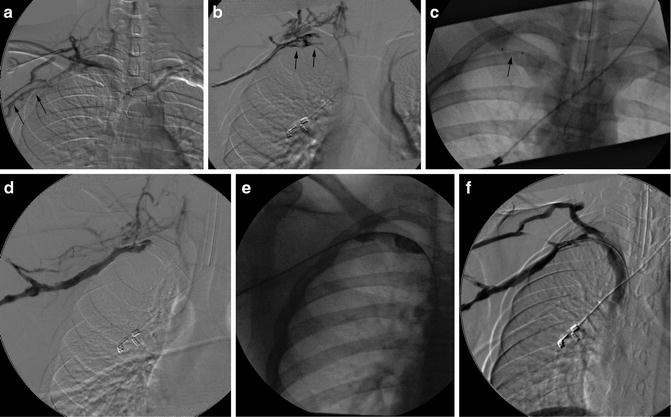

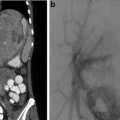
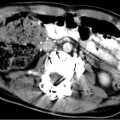
Fig. 15.4
May-Thurner: Fifteen-year-old female with acute left leg swelling, recent change in oral contraceptives, and multiple family members with DVT and PE. (a) Femoral venogram obtained prior to IVC filter insertion demonstrates free floating IVC thrombus (arrows) arising from left common iliac vein. An IVC filter was placed using a jugular approach. (b, c) DSA venography shows left iliofemoral thrombosis (patient is prone). (d) Near-complete thrombus resolution following AngioJet power-pulse spray thrombolysis and thrombectomy. (e) After TPA infusion, thrombosis has cleared but normal antegrade flow through the common iliac has not been reestablished. (f) CT demonstrates external compression of right CFA on left CFV (arrow) consistent with May-Thurner syndrome. (g) Several episodes of unsuccessful venoplasty were undertaken prior to placing a 14 mm wall stent (h)

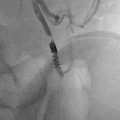
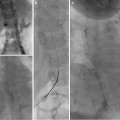
Fig. 15.5
Paget-Schroetter: Fourteen-year-old baseball player with 3-day history of right arm pain and swelling. (a, b) Axillary and subclavian vein thrombosis (arrows) demonstrated on venogram. (c) Power-pulse spray thrombolysis and AngioJet© thrombectomy performed resulting in (d) near-complete clearance. (e, f) Venoplasty was performed resulting in reestablishment of flow. The patient was referred for urgent surgical assessment but required two further episodes of thrombectomy and venoplasty
The use of a wire and catheter used to perform angiography or venography can improve treatment outcomes and can be predictive of success. Rotation of the wire and/or catheter can be used to fragment the thrombus to increase surface area available to react with the lytic agent. The ability to pass a wire through an area of arterial thrombosis is a predictor of success in arterial interventions [55]. This underlines a basic tenet of thrombosis treatment: success is dependent upon the ability to establish both inflow and outflow.
Initial Lytic Administration
When not proceeding directly to thrombectomy, thrombolytic agents can be administered using lacing or pulse spray techniques or with some thrombectomy devices. During the initial administration, single or multiple doses of lytic agent are given. Multiple doses are administered at 15–30 min intervals (to allow time for the lytic agent to act).
Lacing is the injection of lytic agent throughout the length of a thrombus (usually through an end-hole catheter). To perform pulse spray thrombolysis, small aliquots are injected under high pressure to force the lytic agent deeper into the thrombus to improve efficacy. Traditional pulse spray is performed with a multi-side-hole catheter (Fig. 15.6). Certain AngioJet catheters perform an automated version called power-pulse spray.
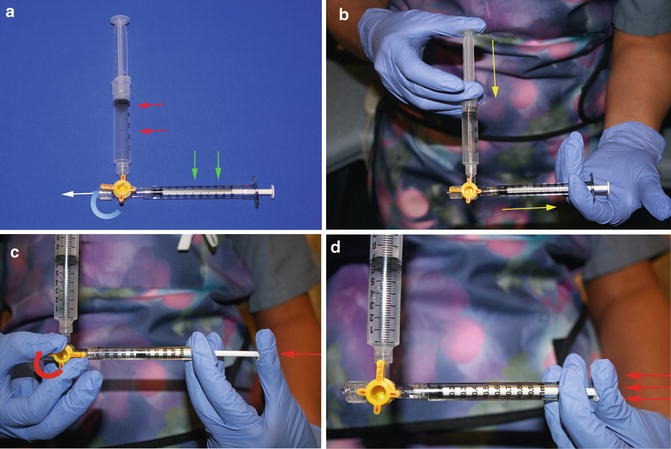

Fig. 15.6
Pulse spray thrombolysis technique. (a) A 3-way stopcock is used to connect a reservoir syringe containing the full dose of TPA (red arrows) and a sturdy, small (1 mL) syringe (green arrows) for injections. The infusion catheter (not shown) attaches at the white arrow. (b) The injection syringe is filled with a small volume (0.1–0.3 mL) of TPA (yellow arrows). (c) Push the plunger of the injection syringe against the closed 3-way stopcock to generate pressure (red arrow). (d) While maintaining pressure, the stopcock is opened causing the plunger of the injection syringe to quickly depress (red arrows), causing TPA to be forcefully ejected through the infusion catheter
Thrombectomy
When used, a thrombectomy device is inserted to disrupt, macerate, or remove thrombus (Fig. 15.5c). The choice of thrombectomy device is dependent upon institutional availability and thrombus location and extent. Venography is used to assess progress and determine if further intervention is necessary (Figs. 15.4d




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree