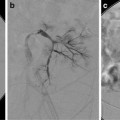
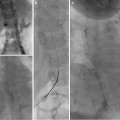
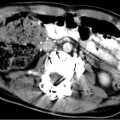
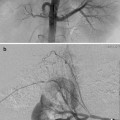
Fig. 25.1
(a) A 15-year-old girl with fibrolamellar HCC. Angiogram performed from the celiac trunk demonstrates the hypervascular character of the tumor involving the left hepatic lobe (arrows). (b) Microcatheter was used to select a dominant feeding artery to the tumor. The chemoembolic emulsion was infused from this selective position. Evident are the oily contrast beads collecting into the tumor. Note a coil was placed into the gastroduodenal artery (GDA) to protect against reflux into the gastric and proximal small bowel supply. (c) Baseline CT demonstrating tumor involving the left lobe. (d) Post-TACE CT without IV contrast demonstrates the distribution of the chemoembolic emulsion throughout the tumor. (e) MRI 3 months following the initial TACE demonstrates tumor necrosis and retraction of the tumor following four rounds of triple drug TACE. There remains residual enhancing tumor (arrows)
Indications and Role of TACE in Children
In that HB is generally chemosensitive, systemic neoadjuvant chemotherapy is typically the preferred initial treatment course prior to or following surgical resection [2]. However, in up to 30 % of children, the tumor will not respond adequately to systemic chemotherapy, and TACE can be considered as an additional treatment option. TACE has been shown to be effective as a salvage preoperative technique to convert unresectable tumor into a resectable tumor [12]. Children that have bilobar or multicentric disease and despite an apparent effective response of the tumor to the systemic or localized regional chemotherapy, surgery may not be able to achieve an adequate resection and still maintain sufficient tissue volume to preserve function. In these circumstances liver transplantation is increasingly considered a viable option, and TACE can serve as a bridging technique until an appropriate organ is available (Fig. 25.2) [13, 14].
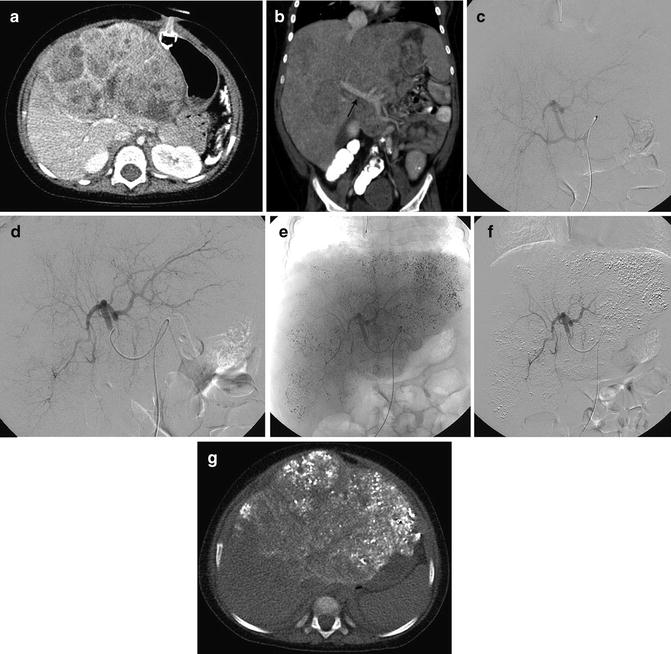

Fig. 25.2
A 23-month-old boy with hepatoblastoma, nonresponsive to systemic chemotherapy, had a TACE performed as a bridge for transplant. The child was transplanted after a single session of TACE when an organ became available. (a) CT demonstrates tumor involving the left hepatic lobe with minimal necrosis (response) to systemic chemotherapy. (b) Coronal image demonstrates patency of the main portal vein (arrow) and extent of left hepatic lobe involvement. (c) Angiogram performed from the celiac trunk. (d) Selective angiogram of the left hepatic lobe demonstrates innumerable enhancing nodules throughout the left lobe. (e) Unsubtracted image demonstrates the distribution of the chemoembolic mixture throughout the nodular masses occupying the left hepatic lobe. (f) Subtracted image demonstrating pruned appearance of the arterial vasculature following particle embolization. (g) Non-contrast CT demonstrating distribution of the chemoembolic mixture within nodules throughout the left lobe and sparing the right
Less than 30 % of children with HCC present with a primarily resectable tumor. Liver transplantation has been shown to be much more successful than conventional systemic chemotherapy in children with HCC, even if they were outside the Milan transplant criteria (adult basis for liver transplantation) [15]. In these circumstances TACE clearly has a role as a bridging technique until an organ is available.
Contraindications
Contraindications are listed in Table 25.1.
Table 25.1
Contraindications
1. Decompensated liver disease with inadequate synthetic function as indicated by: |
(a) Jaundice |
(b) Encephalopathy |
(c) Refractory ascites |
2. Persistent biliary obstruction or untreated varices at risk for hemorrhage |
3. Extensive bilobar tumor infiltration and replacement with tumor (>50 % of liver; inadequate volume to preserve hepatic function) |
4. Inadequate portal vein flow (portal vein occlusion is not a contraindication if there is sufficient cavernous portal flow) |
5. Technical contraindications |
(a) Contraindication to angiography (see Chap. 4) |
(b) Arteriovenous shunting that would result in embolization to the lung |
6. Renal insufficiency (creatinine >2 mg/dL or creatinine clearance <30 mL/min) |
There are three major categories that relate to contraindications for TACE. First are those issues related to the angiographic procedure (see Chap. 4). Second are contraindications related to the chemotherapeutic agents such as severe thrombocytopenia, neutropenia, and cardiac or renal dysfunction. Finally, there are issues related to hepatic function and vascular involvement. The child must have adequate reserves and sufficient hepatic function following the embolization of the hepatic artery. This requires adequate portal venous support at baseline. An occluded portal vein is not necessarily a contraindication to TACE as long as there is sufficient collateral flow from cavernous transformation to support the liver should the arterial supply become compromised as is the case with an effective TACE (Fig. 25.3). In these circumstances the tumor should be embolized as selectively as possible to minimize risk of inducing hepatic failure. Children with biliary obstruction should have this relieved prior to a TACE due to the increased risk of developing a post-TACE hepatic abscess, sepsis, or hepatic failure [18]. Tumors that have excessive arterial venous shunting may not be appropriate for TACE treatment due to the risk of developing clinically significant pulmonary emboli as a result of the embolic material.
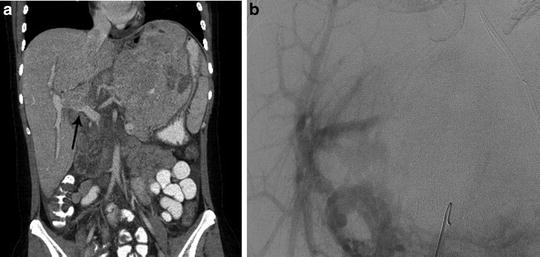

Fig. 25.3
Child with HCC involving the left lobe of the liver and portal vein thrombosis. (a) Coronal CT image demonstrates portal vein occlusion from tumor invasion (arrow). (b) Mesenteric angiogram imaged during the portal venous phase confirms portal vein occlusion, but demonstrates adequate portal supply to the right lobe secondary to cavernous transformation
Preprocedure Workup
The workup before the actual procedure is an extremely important step in the entire process. This includes not only imaging and laboratory values but also a frank discussion at tumor board and a clinic visit for the child and family.
Typically, when a patient is to be worked up for a chemoembolization procedure, by the time referral is made to the interventional radiology service, a definitive diagnosis along with the necessary imaging and lab workup has been completed. However it must be kept in mind that the imaging and labs should be relatively recent prior to the procedure. The imaging modality preferred is usually a multiphase contrast-enhanced MRI. This is especially helpful compared to a CT in the event that there has been prior percutaneous therapy. However if an MRI is not available or obtainable for various reasons, then a multiphase CT with contrast is sufficient.
Along with the imaging, the bloodwork is undertaken to assess for ability to tolerate TACE. The standard bloodwork performed should include a CBC, liver and renal function, coagulation profile, serum electrolytes, and tumor markers. A differential white count and an absolute neutrophil count may be required for those children previously treated with systemic chemotherapy. Once these facts are in hand, the case is discussed in the multidisciplinary tumor board. This is the forum to clarify the ultimate goal of the TACE, i.e., whether this is being done as a primary preoperative procedure, salvage, bridge to transplant, or palliative procedure.
Having made the decision to offer a TACE, the candidacy of the patient needs to be determined. This is done based on the performance status of the patient, the imaging findings, and the lab values. Usually patients with an Eastern Cooperative Oncology Group (ECOG) Performance Status score of more than 2 are poor candidates (see Table 25.2). However, ECOG scoring is always challenging in the pediatric population. On imaging one needs to ascertain the degree of the liver parenchyma replaced. If more than 70 % of the liver is replaced by tumor, then the patient is a poor candidate in terms of tolerating the procedure due to the risk of inducing hepatic failure. Tumor distribution, i.e., unilobar or bilobar, also plays a role in the planning of the procedure. Bilobar disease will need good documentation of the areas treated at each session to make sure that all lesions are treated. Unilobar disease consisting of a solitary lesion is the best scenario as it allows a more targeted delivery of the chemoembolic agent with the best results.
Table 25.2
Eastern Cooperative Oncology Group (ECOG) Performance Statusa
Grade | ECOG |
|---|---|
0 | Fully active, able to carry on all pre-disease performance without restriction |
1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work |
2 | Ambulatory and capable of all self-care but unable to carry out any work activities. Up and about more than 50 % of waking hours |
3 | Capable of only limited self-care, confined to bed or chair more than 50 % of waking hours |
4 | Completely disabled. Cannot carry on any self-care. Totally confined to bed or chair |
5 | Dead |
Next, one needs to assess the lab values. The coagulation parameters and the total bilirubin are critical values to consider. A total bilirubin of more than 2 mg/dL can suggest increased risk for complications, and proceeding with treatment should be taken into careful consideration with the care team. Also remember that when using Adriamycin, since it has cardiac side effects, it requires that cardiac function be evaluated in advance. Some of the lab value cutoffs are mentioned in the Table 25.3.
Table 25.3
Lab values for TACE
1. T. Bilirubin less than 2 mg/dL |