Thyroid Cancer Imaging with PET and SPECT
Steven M. Larson
Ravinder K. Grewal
Michael M. Tuttle
Well-differentiated thyroid cancer includes papillary follicular and Hürthle cell cancers. In the United States, 25,690 new cases are expected in 2005, which is more than double the incidence of the early 1990s. Clinical management of these patients depends on their risk for recurrence, and the majority of tumors, particularly those that are small and confined to the thyroid gland, may be treated with surgery alone. Nuclear medicine has been a mainstay in the management of patients at moderate to high risk for recurrence since its earliest beginnings in the late 1940s. Surgery, followed by iodine 131 (131I) for ablation of thyroid remnant, is the standard of care for these patients. 131I is the mainstay of treatment for well-differentiated metastatic thyroid cancer. Recent improvements in thyroid cancer management have depended on the clinical availability of recombinant TSH (Thyrogen)-sensitive assays for thyroglobulin (TG) and positron emission tomography (PET), especially integrated PET and computed tomography (CT). PET-CT has proven to be extremely useful in higher-risk patients for staging, identifying potentially resectable tumor, and defining the prognosis (see Fig. 37.5). In the future, dosimetry with iodine 124 (124I) will offer lesion-specific dosimetry.
Medullary thyroid cancer (MTC) is less common, but its treatment benefits from diagnostic imaging with indium 111 (111In) octreotide, iodine 123 (123I) metaiodobenzylguanidine (MIBG), and fluorodeoxyglucose (FDG). In both differentiated thyroid cancer and MTC, single-photon emission computed tomography (SPECT) imaging is particularly useful after therapy of both well-differentiated thyroid cancer with 131I and medullary thyroid cancer with 131I-MIBG.
Histologic Classification of Thyroid Cancer
The American College of Surgeons Commission on Cancer conducted a patient care evaluation study of thyroid cancer. This prospective study involved a cohort of thyroid cancer patients admitted to more than 1,500 reporting hospitals in 1996. The histologic classification of thyroid cancers was as follows: 81% were papillary, 10% follicular, 3.6% Hürthle cell, 0.5% familial medullary, 2.7% sporadic medullary, and 1.7% undifferentiated/anaplastic. About 50% of the cohort had ancillary 131I therapy (1). From a nuclear medicine point of view, this breakdown is important because only papillary, follicular, and Hürthle cell cancers are capable of concentrating radioactive iodine. These cancers make up the group of differentiated thyroid cancers.
Differentiated Thyroid Cancer
Incidence and Prevalence
Differentiated thyroid cancer originates from cells within the thyroid follicle, usually presents as a painless lump in the thyroid gland, and is most often detected at the time of
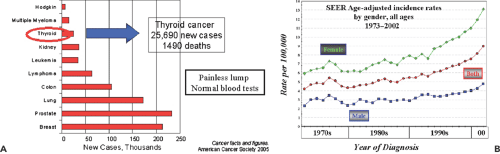
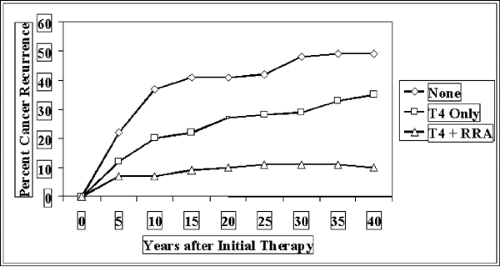
routine physical examination. For reasons that are largely unknown, thyroid cancer incidence is increasing rapidly, and it was predicted there would be 25,690 new cases in the United States during 2005. The total rate of death is not increasing, however, and only 1,490 cases within this cohort were predicted to die (Fig. 37.1). Another interesting feature of this disease is that its course is often relatively long, stretching over many years. For this reason, the prevalence of patients with thyroid cancer is relatively large, and in the United States it is estimated that 327,000 patients are survivors of thyroid cancer; of this group, 47,000 were diagnosed more than 30 years ago. Thyroid cancer is a tumor type that tends to recur (Fig. 37.2). More than 30% of patients will ultimately have recurrent cancer, about 20% by 10 years post-treatment, but with recurrence continuing out to nearly 30 years in some patients.
routine physical examination. For reasons that are largely unknown, thyroid cancer incidence is increasing rapidly, and it was predicted there would be 25,690 new cases in the United States during 2005. The total rate of death is not increasing, however, and only 1,490 cases within this cohort were predicted to die (Fig. 37.1). Another interesting feature of this disease is that its course is often relatively long, stretching over many years. For this reason, the prevalence of patients with thyroid cancer is relatively large, and in the United States it is estimated that 327,000 patients are survivors of thyroid cancer; of this group, 47,000 were diagnosed more than 30 years ago. Thyroid cancer is a tumor type that tends to recur (Fig. 37.2). More than 30% of patients will ultimately have recurrent cancer, about 20% by 10 years post-treatment, but with recurrence continuing out to nearly 30 years in some patients.
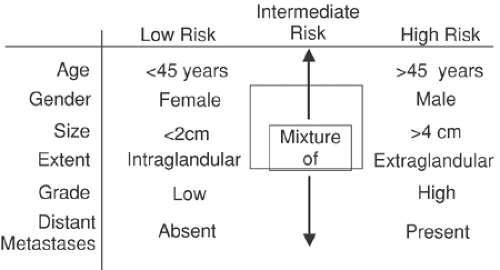
The concept of low- and high-risk tumors is a useful one in well-differentiated thyroid cancer (Fig. 37.3). The AMES categorization uses age, presence of metastases, extension outside the thyroid gland, and size of tumor greater than 4 cm to differentiate between high and low risk, and the low-risk subpopulation has a survival rate of 98% at 20 years, compared with 54% for the high-risk group (2). Nuclear medicine approaches are most useful in the intermediate- to high-risk group.
Based on the natural history of thyroid cancer, modified by the risk factors described above, the take-home message that can be conveyed to the newly diagnosed and treated thyroid cancer patient is as follows: long-term survival is expected, recurrences do occur until years later, and lifelong follow-up is necessary to detect and properly treat any recurrences that may develop.
A detailed discussion of staging of thyroid cancer according to the AJCC TNM classification is outside the scope of this chapter, but a concise and well-written synopsis is available on the Web at this address: http://www.meb.uni-bonn.de/cancer.gov/CDR0000062913.html#REF_14 (3). For well-differentiated thyroid cancer, age is such a dominant factor in prognosis that patients younger than 45 years can achieve a maximum categorization of stage II, even with metastatic disease. On the other hand, patients older than 45 with extension of cancer locally in the neck obtain a stage IV categorization.
Standard Workup in Nuclear Medicine
Thyroid nodules are almost always discovered on physical examination or as an incidental finding on diagnostic imaging for other purposes. The use of radiotracers for evaluating such thyroid nodules is mostly of historical interest in our practice. The reason for this is that fine needle aspiration (FNA) with or without ultrasound guidance provides much more definitive information about the nature of a thyroid nodule.
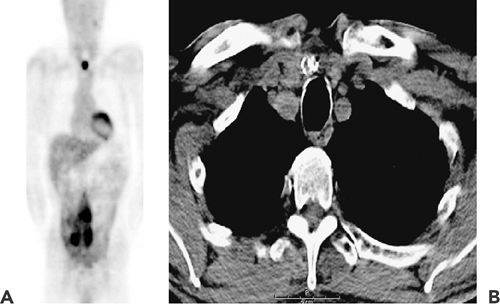
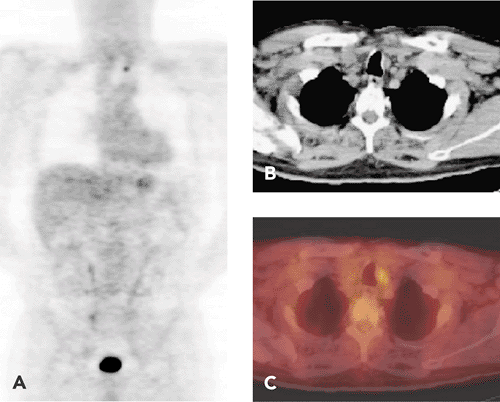
A certain number of thyroid cancers are incidentally detected as thyroid nodules on positron emission tomography (PET) scanning. In patients being screened for other purposes, particularly within a large oncology practice, it is not uncommon to discover hypermetabolic thyroid nodules on fluorodeoxyglucose (FDG)-PET scans (4). Among 4,136 subjects who had undergone PET scanning for other purposes and whose scans were reviewed for the presence of diffuse or focal FDG uptake, 2.2% had increased thyroid uptake that was incidentally discovered. Of these cases of thyroid uptake, half were focal and half diffuse. Among the patients with diffuse uptake, the majority were shown to have a chronic inflammatory condition of the thyroid. Among those with focal uptake, a neoplasm was diagnosed in the biopsied cases, and half of these masses were malignant. The authors concluded that it is important to follow up with biopsy confirmation for any focal abnormality incidentally discovered in the thyroid during the course of FDG-PET imaging (Fig. 37.4).
Treatment with 131I
Ablation of Postsurgical Remnant
Patients with intermediate to high risk of well-differentiated thyroid cancer are referred for ablation of thyroid remnant after total thyroidectomy. This practice has been shown to be effective in reducing the probability of recurrence and mortality (5). Outcome was compared in 1,004 patients with well-differentiated thyroid cancer, including a 151 patients who underwent ablation with 131I. In the 131I-treated group, tumor recurrence was threefold lower (p <.001), and in patients older than 40 years of age at time of diagnosis with primary nodules greater than 1.5 cm, there was a significant reduction in the risk of distant metastases (p <.002) and death (p <.001). The effective dose of 131I was examined by separating the 131I-treated patients into two cohorts: the 43% of patients who received 29 to 50 mCi (mean, 47 mCi) and the 57% who received 51 to 200 mCi (mean, 111 mCi). At mean follow-up of 14.7 years, there was no difference in the rate of recurrence in the patients treated with the two different dose ranges.
General Management and Diagnostic Imaging Evaluation of the Post–Total Thyroidectomy Patient
In the last 10 years, diagnosis and management of thyroid cancer in the post–total thyroidectomy setting has been modified by several important advances: (a) the widespread use of serum thyroglobulin assays for monitoring treatment response and detecting recurrent disease, (b) the growing use of recombinant TSH as a replacement for induced hypothyroidism prior to 131I administration, and (c) the use of PET-CT in moderate- to high-risk patients for staging and monitoring treatment response (Fig. 37.5). 131I continues to be the mainstay of thyroid cancer therapy, as it has been for more than 50 years (6).
There are numerous approaches to the management of the thyroid cancer patient. A practice pattern that has
developed at Memorial Sloan-Kettering Cancer Center is described in Figure 37.5. Typically, the postsurgical patient will be treated with either 100 mCi (no soft-tissue extension or metastatic lymph nodes) or 150 mCi (metastasis or other adverse prognostic features). Once patients have been referred for ablation with 131I, they are followed for an extended period of time, with more extensive testing at 1 year postablation. At the time in their annual testing, if metastatic disease is discovered, they will be re-treated, typically with doses varying from 200 to 500 mCi of 131I (Fig. 37.6). In order to treat at such high doses safely, we have established a procedure for estimating dosimetry of the whole body and blood and bone marrow. We plan dosing so that the total radiation exposure in any single administration does not exceed 200 cGy to the blood. This dose is approximately equivalent to 80 cGy to the bone marrow. The details of and rationale for this approach have been described (7).
developed at Memorial Sloan-Kettering Cancer Center is described in Figure 37.5. Typically, the postsurgical patient will be treated with either 100 mCi (no soft-tissue extension or metastatic lymph nodes) or 150 mCi (metastasis or other adverse prognostic features). Once patients have been referred for ablation with 131I, they are followed for an extended period of time, with more extensive testing at 1 year postablation. At the time in their annual testing, if metastatic disease is discovered, they will be re-treated, typically with doses varying from 200 to 500 mCi of 131I (Fig. 37.6). In order to treat at such high doses safely, we have established a procedure for estimating dosimetry of the whole body and blood and bone marrow. We plan dosing so that the total radiation exposure in any single administration does not exceed 200 cGy to the blood. This dose is approximately equivalent to 80 cGy to the bone marrow. The details of and rationale for this approach have been described (7).
When a patient has extensive metastatic disease, it is important to characterize the localization as a guide to further therapy. Because of the very large dose of 131I that is administered, there is often excellent diagnostic information to be obtained from a whole-body scan performed 5 to 7 days following the administration. In Figure 37.5B, planar gamma camera images are seen after 250 mCi of 131I. There is extensive uptake in the thorax, including the lungs, as well as in the axial skeleton and extremities. SPECT is often useful in this situation, as a means of defining the extent of the tumor and in particular its relationship to vital bony structures (Fig. 37.7).
In recent years, an important component of our approach has been to use recombinant TSH for the purpose of preparing patients for treatment, either ablation (7) or therapy (8). We have shown that for diagnosis, ablation, and 1-year outcome of therapy (Fig. 37.8), recombinant TSH is equivalent to hypothyroid preparation for radioiodine therapy. Of course, recombinant TSH is much better tolerated by the patient, and hypothyroid side effects are avoided. It should be noted that these side effects can be severe and may include both somatic and mental dysfunction. For example, hepatic enzyme alteration is common in severe hypothyroidism, and psychotic episodes may be seen. Patients whose jobs require hard physical labor or high mental acuity may be unable to work for several weeks while in the hypothyroid state.
Integrating PET-CT into Patient Management
As FDG-PET became more widely utilized, it was recognized that some patients with thyroid cancer had positive PET scans (9,10,11,12,13,14,15,16,17,18). There is now no doubt that PET adds significantly to the improvement of thyroid cancer management (19). Patient selection is critical if one is to optimize the use of PET in thyroid cancer. The most common indications for PET are an elevated TG level together with a negative whole-body radioiodine scan (20); a histologic diagnosis of Hürthle cell cancer (21); a restaging of known metastasis (22); adverse risk factors, such as biologically aggressive histology (e.g., tall cell papillary thyroid cancer); and advanced age (23).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree