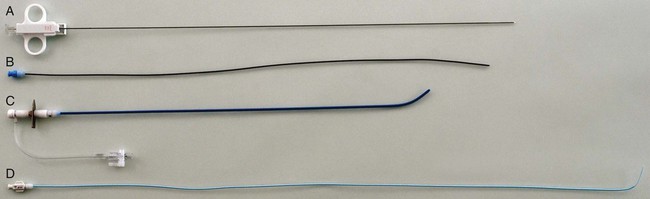
Sebastian Kos, Deniz Bilecen, Augustinus Ludwig Jacob and Markus H. Heim Historically, Paul Ehrlich, a German pioneer of immunology, was the first to perform a percutaneous liver biopsy. In 1883, he determined the hepatic glycogen content in a diabetic patient.1 In the following years, Lucatello applied the technique on a liver abscess (1895). Schüpfer et al. had already documented its potential in the diagnosis of chronic cirrhotic liver disease in a series of rat experiments and clinical studies. After Menghini propagated the “1-second needle biopsy of the liver” 50 years later, this procedure was broadly implemented in the management of chronic and acute hepatic diseases.2 Therefore, alternative approaches (laparoscopic, transfemoral, transjugular) were established, enabling hepatic tissue sampling after failure of, or in the presence of contraindications for, the biopsy procedure.3 Given their higher mortality and morbidity, the use of laparoscopic biopsies is reduced to special cases, such as when an abdominal operation under general anesthesia is mandatory otherwise.3 As a pioneer, Dotter was the first to biopsy dog livers using a transvenous jugular approach in 1964.4 Weiner and Hanaffe used the same access to catheterize the hepatic veins in 1967, and in 1970 published their initial experience with transjugular hepatic biopsy in a series of humans.5,6 By 1973, Rosch had already performed the technique on 44 patients, showing the need for the procedure.7 Unlike percutaneous access, it approaches the liver through the right jugular vein, through the vena cava, and most often the right hepatic vein. The biopsy is then performed directed away from major vessels into the parenchyma (inside-out). The capsule is not injured, minimizing the risk of intra- or perihepatic hematoma. Blood leakage into the biopsy channel immediately drains into the access vein, thereby avoiding significant extravascular blood loss and hemodynamic alterations. Today the transjugular liver biopsy is well accepted and broadly established as the first-line backup for the percutaneous approach.8 In the hands of an experienced interventional radiologist, it is safer than the latter and well tolerated. Since many patients with liver disease consequently suffer from coagulopathy and/or ascites, there is huge potential for the technique. Optional concomitant measurement of the hepatic venous pressure gradient (HVPG) is calculated as the difference between wedged (WHVP, an estimate of portal venous pressure) and free hepatic venous pressure (FHVP). It was shown that the HVPG provides relevant information to assess the risk of esophageal varix bleeding in patients with known portal hypertension. Furthermore, it may help document the efficacy of drug treatment in those patients by its changes over time.9 The risk of variceal bleeding is zero with HVPG lower than 12 mmHg, whereas an HVPG of more than 20 mmHg in cirrhotic patients is predictive of early rebleeding and death.9 The following general indications for liver biopsy in diffuse nonfocal liver disease were modified from Bravo et al.3: • Diagnosis, grading (inflammatory activity), and staging (degree of fibrosis/cirrhosis) of noninfectious liver diseases (e.g., nonalcoholic steatohepatitis [NASH], alcoholic liver disease, autoimmune hepatitis) • Grading and staging of infectious liver diseases (e.g., chronic hepatitis B or C) • Diagnosis of hemochromatosis, with quantitative analysis of iron levels • Diagnosis of Wilson disease, with quantitative analysis of copper levels • Diagnosis of primary biliary cirrhosis and primary sclerosing cholangitis • Detection of adverse effects occurring under drug treatment (e.g., methotrexate) • Evaluation of abnormal biochemical tests in combination with negative or inconclusive serologic testing • Evaluation of liver status after transplantation and of the donor liver before transplantation Transjugular liver biopsy is indicated in the following specific circumstances: • Severe coagulopathy (thrombocytopenia < 70,000; prothrombin time < 60% of normal level) • Suspected vascular tumor or peliosis hepatic • Need for additive vascular procedures like transjugular intrahepatic portosystemic shunting • Need for additive biopsies (e.g., kidney) • Need for additive diagnostics (e.g., venography, HVPG) • Failure of prior percutaneous liver biopsy For transjugular liver biopsy, different biopsy needles have been designed. Corr et al. reported a series of 200 cases which were biopsied with a modified Ross needle (Cook Medical, Bloomington, Ind.). This was placed through a 9F curved sheath.11 More often encountered are side-cutting needles like the Quick-Core needle (Cook Medical), which is inserted using a 7F curved sheath with a stiffening and guiding metal cannula. The cutting needles available (18 and 20 gauge) provide a semiautomated springfire mechanism to acquire tissue. This, according to our own experience and the literature, is relatively easy to use and has a high diagnostic yield. In 43 patients, Little et al. performed successful biopsy in 98% (42/43 patients)12; 118 biopsies were obtained, with an average of 2.8 passes per patient. Mean maximum sample lengths lay between 1.1 and 1.5 cm. Other studies showed successful biopsy rates of 100%, with 100% diagnostic histologic core specimens.13 Recently a new aspiration needle device (Hakko Co. Ltd., Nagano, Japan) was evaluated in Niigata, Japan, with promising initial results compared to the Quick-Core needle (less fragmentation, longer specimens, more portal triads, shorter intervention) but is not yet commercially available.13 Key equipment (Fig. 63-1) includes:
Transjugular Liver Biopsy
Clinical Relevance
Indications
General Indications for Liver Biopsy in Diffuse Nonfocal Liver Disease
Specific Indications for Transjugular Liver Biopsy
Equipment
Transjugular Liver Biopsy