SONOGRAM ABBREVIATIONS
Bl Bladder
D Diastole
K Kidney
R Reversed flow
S Systole
KEY WORDS
Acute Tubular Necrosis (ATN). Acute renal shutdown, usually due to abrupt decrease of blood pressure.
Anuria. Total absence of urine production.
Creatinine. A waste product excreted in the urine. Elevated values may indicate that the patient is in renal failure.
Cyclosporine. Drug given to transplant recipients to prevent rejection; may cause a clinical picture resembling rejection.
Iliac Fossa. Area on either side of the lower part of the abdomen. Usual location of a transplanted kidney.
Immunosuppression. Suppression of host’s immunologic defenses.
Infarct. Dead tissue due to lack of blood supply.
Ischemia. Decrease in blood supply. Prolonged ischemia may result in an infarct.
Lymphocele. A collection of lymphatic fluid. Usually a postoperative complication.
Oliguria. Decreased urine production.
Rejection. Reaction of the body’s immune system to the presence of a foreign kidney.
Steroids. Drugs similar to the hormones produced by the adrenal glands. Steroids lead to a suppression of the immunologic response.
Urinoma. A collection of extravasated urine, usually a postoperative or ischemic complication.
RELEVANT LABORATORY VALUES
Creatinine (serum): Normal is less than 1.5 mg/dL. Urine output: Less than 200 mL in adults (<15–20 mL/kg in children) per 24 hours is abnormally low.
The Clinical Problem
Renal transplantation has been performed since the 1950s and has become the usual long-term treatment for end-stage chronic renal failure. In the last few years, results have improved considerably with the development of effective immunosuppressive medication and better tissue typing, decreasing the risk of rejection. Often these patients will present with symptoms of worsening renal failure. It is helpful to obtain as much clinical history from the patient before starting the ultrasound examination. Obtaining information such as date of transplant, location of transplant, type of transplant, and relevant laboratory value information can help the sonographer and sonologist to tailor the ultrasound examination to answer the clinical question.
Indications for renal transplant ultrasound include worsening renal failure, decreased urine output, elevated serum creatinine, suspected rejection, fever, bruit, flank pain, and fluid leaking from wound. The most common indication to examine a renal transplant is worsening renal failure. Possible explanations include obstruction, rejection, acute tubular necrosis (ATN), and vascular problems such as infarct or venous thrombosis. Renal transplant rejection, the usual cause of fever and increasing serum creatinine levels, has a typical although far from diagnostic ultrasonic appearance. Hydronephrosis and vascular problems can be diagnosed by ultrasound. Computed tomography and nuclear medicine are other imaging modalities that may be used, but ultrasound is generally the most useful first test for most patients.
Another common clinical problem in a renal transplant recipient is postoperative fever, which may be due to an infected fluid collection. Types of collections are hematomas, lymphoceles, and urinomas. All of these collections are easily localized by ultrasound and can be drained or aspirated under ultrasound guidance. Because transplant recipients are immunosuppressed, serious infections may produce few warning signs and infection may spread rapidly.
Ultrasound is also a useful tool to guide renal biopsy. This procedure is usually done when there is difficulty in distinguishing rejection, ATN, and cyclosporine toxicity.
The site for placement of a transplant kidney is determined by several factors, including presence of a previous transplant graft, asymmetry in peripheral vasculature, presence of polycystic kidney disease, and preference to perform the transplant in a fashion that does not require crossing of the renal artery and renal vein. This can be done by transplanting the kidney to the same side that it was removed from. However, if the internal iliac artery is used for the arterial anastomosis, the artery and vein do not cross when situated in the contralateral iliac fossa. The renal artery is attached to either the internal or the external iliac artery. The ureter is inserted into the bladder above the ureteral orifice through a submucosal tunnel in the bladder wall. This tunnel creates a valve in the distal end of the ureter to prevent reflux into the renal transplant. Because operative procedures and recipient shape and size vary, the transplanted kidney may have a large renal pelvis or an unusual axis. A baseline ultrasound scan after surgery is therefore sometimes worthwhile to document renal size, pelvicaliceal pattern, and any perirenal fluid collection. Other variations on the surgical procedure include pediatric en bloc transplants (paired kidneys and aorta donated from a young child, with the two kidneys compensating for the fact that the kidneys are smaller than usual) and transplanting two somewhat poorly functioning adult kidneys into an adult, one in each iliac fossa, to compensate for the decreased function of each kidney with an extra kidney.
Anatomy
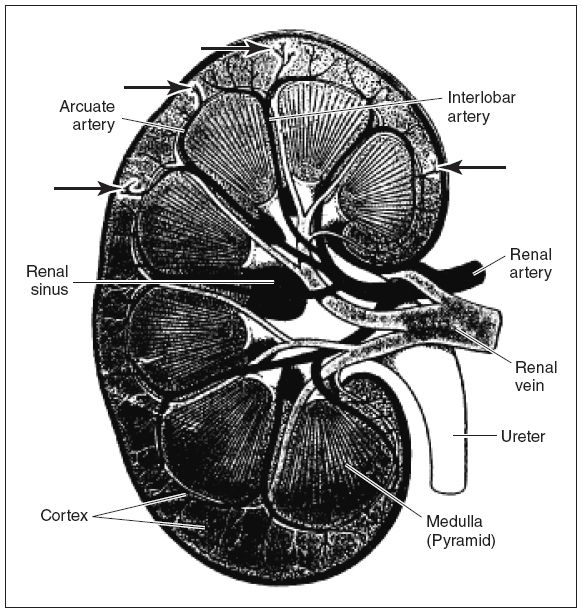
The kidney parenchyma has two main components: the cortex, in which arteries, veins, convoluted tubules, and glomerular capsules are found; and the medulla, which contains the renal pyramids. These are conical masses with papillae projecting into cuplike cavities in the renal pelvis. The main renal artery is located at the hilum, the segmental artery lies adjacent to the medulla, the interlobar artery runs alongside the pyramids, and the arcuate arteries are found at the apex of the pyramids. The interlobular (or cortical) arteries carry blood from the apex of the pyramid to the cortex. The veins parallel the arteries. Arterial anatomy must be understood before undertaking Doppler investigation of the kidneys because this is a very important part of the renal transplant ultrasound examination (Fig. 22-1). In renal transplant recipients, often the kidney is placed in the right iliac fossa. In cases in which a pancreas is also transplanted or if this is a second renal transplant for the patient, the renal transplant may be located in the left iliac fossa.
Figure 22-1. ![]() Details of renal arterial flow. Doppler is usually performed at the main renal artery, interlobar artery, and arcuate artery. Interlobular (cortical) arteries (arrows without labels).
Details of renal arterial flow. Doppler is usually performed at the main renal artery, interlobar artery, and arcuate artery. Interlobular (cortical) arteries (arrows without labels).
Technique
Begin by selecting the transducer with the highest frequency that allows for adequate depth penetration. Most renal transplants can be imaged with a 5-MHz transducer. A curved linear transducer will provide a large field of view in the near zone, so that accurate length measurements may be made without cutting off part of the kidney on either end of the image. Newer developments such as extended field-of-view technology or sector width extension make it even easier to accurately measure large kidneys. Once the transducer has been selected, begin scanning along the long axis of the scar. The scar provides a landmark for beginning to look for the transplant kidney. The long axis of the kidney is usually oriented along the long axis of the scar and the transverse kidney images will sit at right angles to the scar (Fig. 22-2). The transplant kidney should be imaged from lateral to medial in the longitudinal plane, paying close attention to note any fluid collections. After the longitudinal survey of the kidney is completed, the kidney should be imaged in the transverse plane and surveyed from superior to inferior pole, again evaluating for any evidence of fluid collections while in the survey mode. Fluid collections may represent hematomas, abscesses, or lymphoceles. The kidney should be measured in both longitudinal and transverse planes (length, height, and width), because size increase is a potential sign of rejection (although in clinical practice this is generally not too useful). Also, while imaging the kidney the sonographer/sonologist should be evaluating the kidney’s echogenicity (cortex, medulla, sinus), the shape of the kidney, and any evidence of urinary obstruction (i.e., dilated renal pelvis).
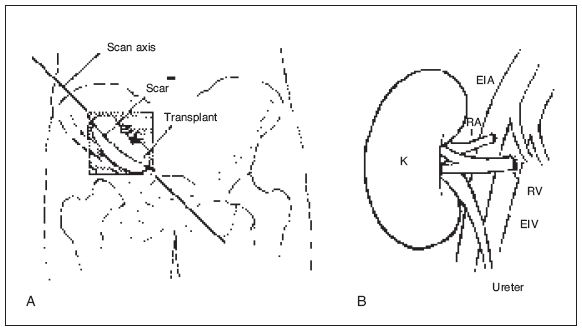
Figure 22-2. ![]() A. Usual placement site of the renal transplant. As a rule, the renal transplant is best shown by scans that are parallel to the scar. The transplant can be in either iliac fossa. B. One of the many types of vascular anastomoses. (B from Jaques BC. Renal transplant surgery. In: Ultrasound of abdominal transplantation. Sidhu PS, Baxter GM, eds. New York: Thieme, 2002:24, Fig. 3-2.)
A. Usual placement site of the renal transplant. As a rule, the renal transplant is best shown by scans that are parallel to the scar. The transplant can be in either iliac fossa. B. One of the many types of vascular anastomoses. (B from Jaques BC. Renal transplant surgery. In: Ultrasound of abdominal transplantation. Sidhu PS, Baxter GM, eds. New York: Thieme, 2002:24, Fig. 3-2.)
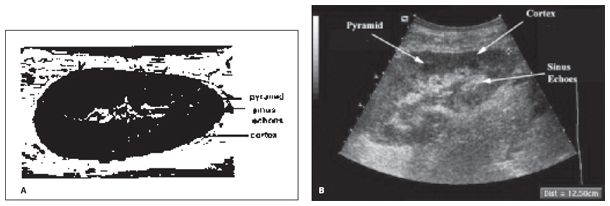
Figure 22-3. ![]() Ultrasound image of a transplant kidney showing the principle anatomic structures. A, diagram; B, sonogram.
Ultrasound image of a transplant kidney showing the principle anatomic structures. A, diagram; B, sonogram.
The urinary bladder should be scanned; if it is filled with urine and there is evidence of urinary obstruction in the kidney, the scan should be repeated with an empty bladder. Hydronephrosis, if present with a full bladder, may disappear with voiding. Doppler signals are obtained in the main renal vein and in the main, segmental, interlobar, and arcuate arteries (Figs. 22-1 to 22-4). The arcuate arteries are sampled in the upper pole, interpolar, and lower poles of the kidney. Color Doppler is helpful in determining the best position and angle in which to place the Doppler gate. Many machines offer a preset for “low flow” states. This setting is helpful to optimize the color Doppler imaging of the kidney. Once a spectral Doppler signal is obtained, the resistive index can be measured. The resistive index should be measured in the arcuate arteries and is calculated from the signal as follows:
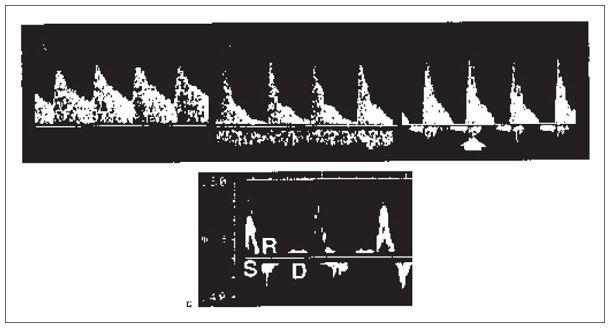
Figure 22-4. ![]() Renal artery Doppler flow pattern. A. Ultrasound image of a normal transplant kidney and main renal artery Doppler. B. Moderate rejection, little diastolic flow; venous flow also seen below the baseline. C. Severe rejection. Reversal of flow in diastole (white arrow). D. Normal iliac artery showing systolic phase (S), reversal phase (R), and diastolic phase (D).
Renal artery Doppler flow pattern. A. Ultrasound image of a normal transplant kidney and main renal artery Doppler. B. Moderate rejection, little diastolic flow; venous flow also seen below the baseline. C. Severe rejection. Reversal of flow in diastole (white arrow). D. Normal iliac artery showing systolic phase (S), reversal phase (R), and diastolic phase (D).
![]()
If the baseline scan is performed, the incision will be fresh and sterile technique should be used. See Chapter 55 for a discussion of how to scan with a gel-film skin barrier or how to cover the transducer.
POWER DOPPLER IMAGING
This technology is particularly helpful in the assessment of renal and pancreatic transplants. It is less dependent on beam angle and is free from aliasing. Perfusion of the transplant can be assessed so infarcts with no flow can be seen. Slower flow can be seen to enable investigation of the venous structures.
Pathology
1. Acute rejection. Acute rejection can occur hours or days after transplantation and has a typical sonographic appearance. The kidney is swollen and the pyramids are more prominent and sonolucent than usual; the central sinus echo complex shows a decrease in both size and echogenicity (referred to as loss of the cortical/medullary junction) (Fig. 22-5). Volumetric changes in renal size can be followed by using the following formula: length × width × height × 0.52 (the formula for a prolate ellipse).
If the Doppler resistive index is greater than 0.75, there is increased vascular resistance due to swelling of the parenchyma. This generally means rejection but can also be seen with other processes that can cause tissue swelling, such as ATN and cyclosporine toxicity, as well as tense perinephric fluid collections, hydronephrosis, or excessive transducer pressure pushing on the kidney.
2. Chronic rejection. Chronic rejection develops months to years after transplantation as a consequence of repeated episodes of acute rejection. This disease results in vascular compromise of the transplant and decrease in renal function. In chronic rejection, the renal parenchyma becomes echogenic and the kidney decreases in size. The vascular resistive index increases. Eventually, the kidney fails.
3. Acute tubular necrosis (ATN). ATN is the most common complication in the first 48 hours after transplantation. Risk factors for ATN include cadaveric graft, hypotension in the donor before transplantation, or long warm (>30 minutes) or long cold (>24 hours) ischemic time periods. Clinically, ATN is difficult to diagnose because it mimics rejection. On ultrasound, the kidney may appear normal or enlarged (enlargement seen with severe disease). If edema is present, the cortex may appear hypoechoic with loss of the normal cortical/medullary interface. If the disease is severe, the renal sinus may appear small due to being compressed by the swollen cortex. With severe ATN, the Doppler resistive index may be greater than 0.75; however, clinically significant ATN may be associated with a normal resistive index as well.
4. Cyclosporine toxicity. Cyclosporine nephrotoxicity occurs when the levels of cyclosporine are elevated, thus compromising renal function. A sonogram usually shows no change in renal size or resistive indices, although resistive indices may be elevated.
5. Hydronephrosis. Obstruction can occur at any time, but commonly occurs in the first few weeks after surgery. There are similar symptoms to rejection. The sonographic characteristics of hydronephrosis (Fig. 22-6) are discussed in Chapter 18. Obstruction may be due to a fluid collection or a poor-quality ureteral anastomosis. A baseline comparison study is important because some pelvic dilatation may be postoperative. Scans over the bladder may show a dilated ureter due to kinking. If the bladder is full, empty it because apparent hydronephrosis may be caused by an overdistended bladder causing kinking of the ureter or reflux.
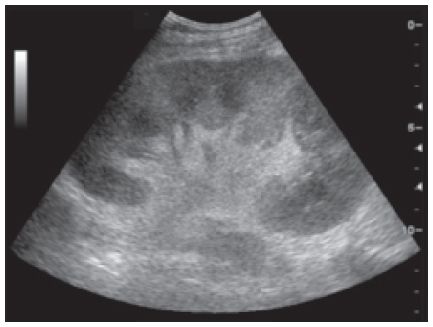
Figure 22-5. ![]() Swollen echogenic kidney associated with acute rejection.
Swollen echogenic kidney associated with acute rejection.
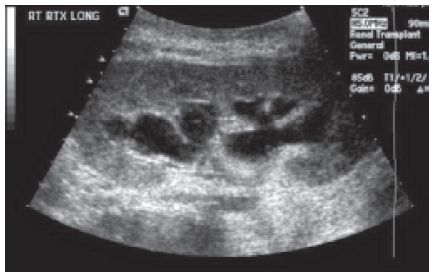
Figure 22-6. ![]() Transplant kidney demonstrating anechoic fluid-filled calyces as seen with hydronephrosis.
Transplant kidney demonstrating anechoic fluid-filled calyces as seen with hydronephrosis.
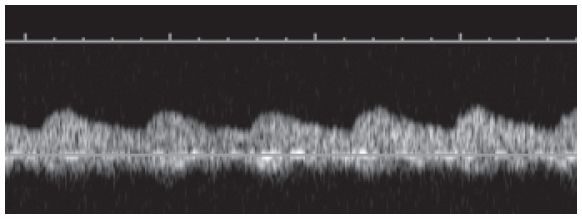
Figure 22-7. ![]() Tardus parvus signal in the arcuate artery distal to a stenosis.
Tardus parvus signal in the arcuate artery distal to a stenosis.
6. Vascular problems.
a. Renal artery stenosis (RAS). RAS is the most common vascular complication and affects renal function. RAS usually occurs within 1 cm of the anastomosis; therefore, it is important to try and see the anastomosis site. This is done by locating the iliac artery and its communication to the main renal artery. The iliac artery will demonstrate a high-resistance Doppler signal (Fig. 22-4D). After the anastomosis site is located, scan the main renal artery thoroughly using both duplex and color Doppler. If the renal artery is stenosed, the Doppler signal will show increased velocity at the site of stenosis (Color Plate 22.1) and a slow increase in systole with delayed acceleration to peak velocity distal to the stenotic site (Fig. 22-7). This waveform is referred to as a tardus parvus waveform (see Renal Artery Stenosis in Chapter 18). Color Doppler may demonstrate aliasing at the site of narrowing, indicative of elevated velocities. After interrogating the main renal artery, sample the arcuate arteries. Obtain a sample in the upper pole, mid-kidney, and lower pole. Again, note any evidence for a tardus parvus waveform, possibly indicating a proximal stenosis.
b. Renal artery thrombosis. Renal artery thrombosis is an unusual complication that sometimes happens in the early postoperative period. Patients often present with anuria and hypertension. Duplex and power Doppler show absent arterial and venous flow within the kidney or a portion of the kidney (an infarct). Angiography may be used to confirm the diagnosis.
c. Renal vein thrombosis (RVT). RVT is a rare complication that usually occurs in the first week after surgery. RVT usually occurs due to extrinsic compression or kinking of the renal vein due to mobility of the graft. These patients usually present with oliguria or anuria and elevated creatinine in the early postoperative period. There will be an enlarged hypoechoic kidney. Both duplex and color Doppler show absent venous flow in the kidney and in the main renal vein and a high-resistance arterial flow with reversed diastolic flow (resistive index >1.00) (Fig. 22-8). If the thrombus is in only the peripheral veins, it will not be seen with ultrasound. Sometimes, the renal sinus echoes are more prominent than usual because of hemorrhage.
d. Renal vein stenosis. Renal vein stenosis can be detected with ultrasound if it occurs in the main renal vein. In most cases, the kidney is enlarged and the renal sinus echoes are more prominent than usual. Doppler analysis reveals increased velocity—three to four times greater than that of the prestenotic vein. Color Doppler shows aliasing at the site of stenosis.
e. Intrarenal arteriovenous (AV) fistulas. Both intrarenal AV fistulas and pseudoaneurysm complications result from biopsy. These are generally benign and usually resolve spontaneously. AV fistulas are easily detected with duplex and color Doppler. Color Doppler shows a localized region of disorganized color that extends outside the normal renal vessels (Color Plate 22.2). Doppler shows a high-velocity, turbulent mass with low resistant arterial and pulsatile venous flow within.
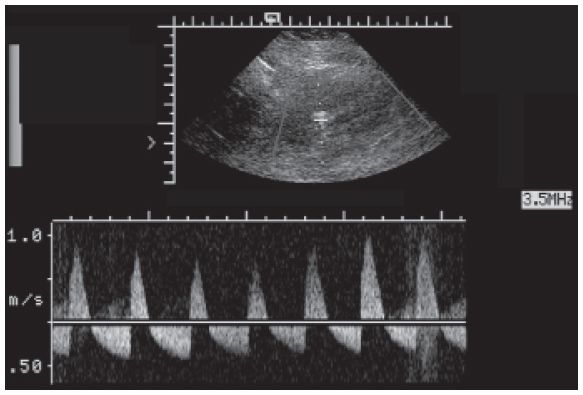
Figure 22-8. ![]() Classic reversed diastolic flow in the intrarenal arterial signal in a patient with renal vein thrombosis (RVT).
Classic reversed diastolic flow in the intrarenal arterial signal in a patient with renal vein thrombosis (RVT).
f. Pseudoaneurysm. A pseudoaneurysm on grayscale looks like a complex or simple cyst; however, color interrogation of the structure demonstrates arterial flow within the cyst. Spectral Doppler will demonstrate to and fro flow in the neck and disturbed flow in the pseudoaneurysm.
g. Renal infarcts. These are usually focal but may involve all of the kidney. Fresh infarcts present as hypoechoic, wedge-shaped areas within the kidney. Power Doppler shows absent flow in the infarcted areas. Occasionally, there may be a focally echogenic area that relates to hemorrhage into the infarct. Eventually, the infarcts decrease in size and a scar develops at the infarct site.
7. Fever or local tenderness of renal transplant. Fever in the posttransplant period may be due to an abscess, hematoma, or, usually, rejection. Because such patients are treated with steroids, they are immunosuppressed, and local tenderness over a collection may be relatively trivial. Whenever hydronephrosis is found, a collection should be sought as its cause.
8. Fluid collections (Fig. 22-9).
a. Hematomas commonly occur in the postoperative patient and are usually located either in the subcutaneous tissues or around the transplant. The sonographic characteristics vary with age; acute (Fig. 22-10A) or chronic hematomas are echogenic and typically hard to distinguish from neighboring structures, whereas intermediate ones (Fig. 22-10B) are complex. The borders are usually well defined. They are often aligned along the renal capsule. Some hematomas are fluid-filled and resemble a urinoma (Fig. 22-10C).
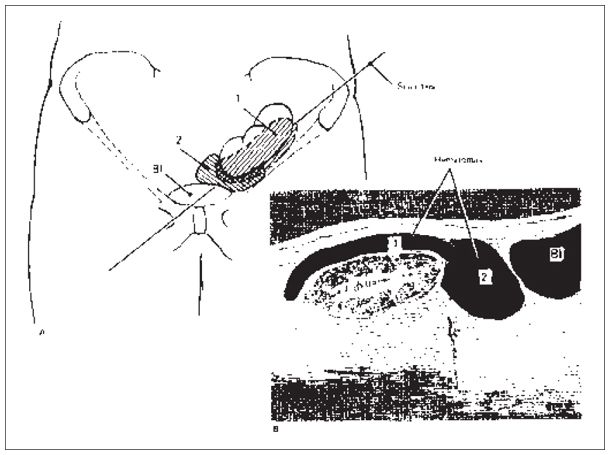
Figure 22-9. ![]() Collections, generally hematomas, are common with renal transplants. Collection 1 is in the usual site of hematoma accumulation. Collection 2 is in a site where urinomas or lymphoceles commonly occur. The axis along which the “sonogram” was performed is shown in (A).
Collections, generally hematomas, are common with renal transplants. Collection 1 is in the usual site of hematoma accumulation. Collection 2 is in a site where urinomas or lymphoceles commonly occur. The axis along which the “sonogram” was performed is shown in (A).
b. Abscesses are suspected when the patient presents with fever and increased white blood count. They can be found in any location and can vary sonographically from an echo-free to a complex echo pattern. They are difficult to distinguish from hematomas by their sonographic appearance.
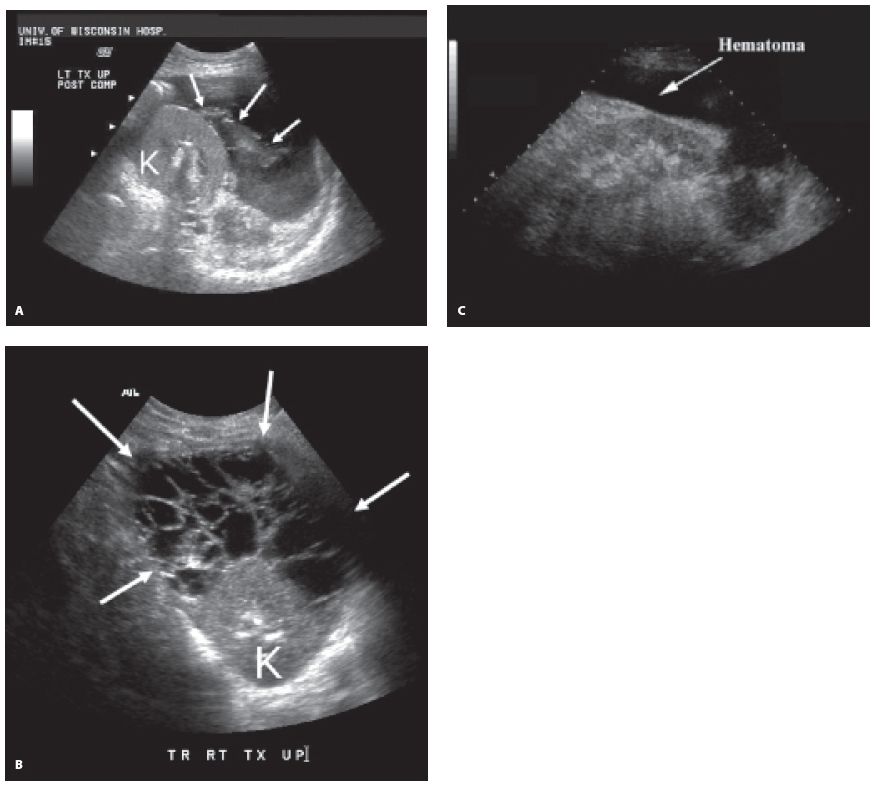
Figure 22-10. ![]() A. Acute bleed immediately after biopsy. Blood jetting from punctured cortical surface (arrows) is easily seen amid surrounding ascites. B. Intermediate age blood shows complex echotexture. C. Chronic hematoma (seroma) demonstrated around the transplant kidney (K) resembles ascites.
A. Acute bleed immediately after biopsy. Blood jetting from punctured cortical surface (arrows) is easily seen amid surrounding ascites. B. Intermediate age blood shows complex echotexture. C. Chronic hematoma (seroma) demonstrated around the transplant kidney (K) resembles ascites.
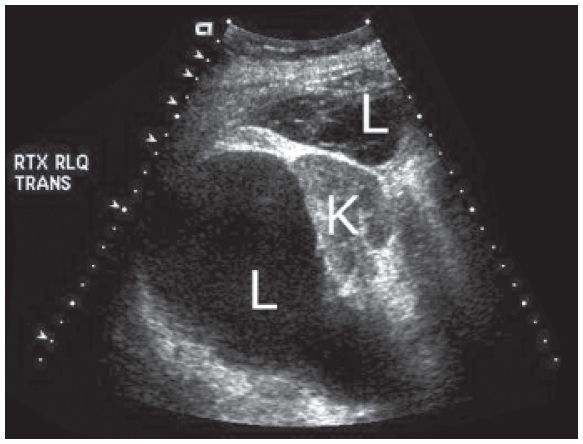
Figure 22-11. ![]() Two well-defined cystic masses, one of which is anechoic and the other of which is slightly complex, representing lymphoceles (L) surrounding the transplant kidney (K).
Two well-defined cystic masses, one of which is anechoic and the other of which is slightly complex, representing lymphoceles (L) surrounding the transplant kidney (K).
c. Lymphoceles (Fig. 22-11) can occur after surgery, especially when there is a blockage or damage of the lymphatic channels. On ultrasound they are usually well-defined, anechoic cystic areas; a majority tend to have septations. Such collections are usually located between the bladder and the kidney. Hydronephrosis due to obstruction by the lymphocele may develop.
d. Urinoma is a serious complication, and it is most commonly caused by an ischemic or surgical problem at the ureteropelvic, ureteroureteral, or ureterovesical anastomosis. Patients usually present with a decrease in urine output, pain, and swelling around the transplant. This type of collection is usually echo-free. Its location is variable, although it is more commonly seen around the lower pole. As always in the presence of a fluid collection, a percutaneous aspiration can determine the cause of the collection. Urinomas, however, can be diagnosed by a nuclear medicine study in a noninvasive fashion.
Pitfalls
1. Pseudohydronephrosis.
a. Bladder overdistention. An erroneous diagnosis of hydronephrosis may be made if the bladder is full. Make sure that a postvoid view is obtained if hydronephrosis appears to be present. Sometimes the apparent hydronephrosis disappears when the bladder is empty.
b. Baseline sinus distention. An incorrect diagnosis of hydronephrosis may be made if a baseline study has not been performed because many transplanted kidneys show some apparent renal pelvic fullness. Long-standing renal transplants often demonstrate a mildly dilated collecting system.
2. Time-gain compensation problems. Poor time-gain compensation settings may give the appearance of a collection anterior to the kidney or even an anterior infarct if the time-gain compensation is too steep (Fig. 22-12).
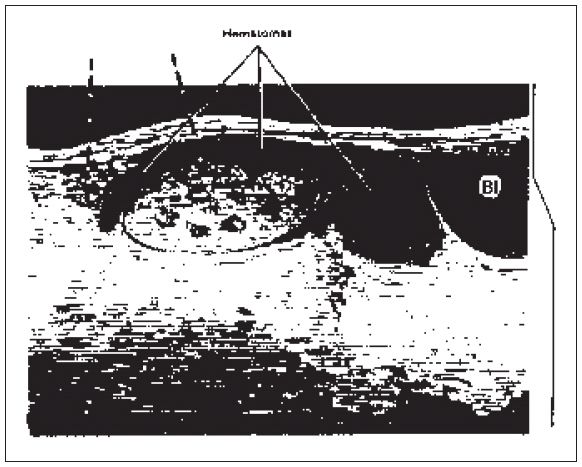
Figure 22-12. ![]() Unsatisfactory time-gain compensation (TGC) settings may prevent assessment of the anterior aspect of the kidney. This hematoma is hard to distinguish from the neighboring tissues because the TGC was too steep (right). TGC artifact is apparent (broken arrows).
Unsatisfactory time-gain compensation (TGC) settings may prevent assessment of the anterior aspect of the kidney. This hematoma is hard to distinguish from the neighboring tissues because the TGC was too steep (right). TGC artifact is apparent (broken arrows).
3. Echogenic collections. Hematomas and abscesses may be missed unless their occasional high echogenicity is kept in mind. If bowel is around, look for peristalsis to differentiate fluid-filled bowel from an abscess or hematoma.
4. Bladder versus other fluid collection. Make sure that an apparent collection below the kidney is not the bladder; ask the patient to void or fill the bladder. Conversely, do not mistakenly attribute an abnormal fluid collection as the urinary bladder. Voiding or placement of a Foley catheter is helpful in this situation.
5. Iliac artery. Do not confuse the iliac artery with the main renal artery. The iliac artery will be outside the confines of the transplant kidney and will show a high-resistance flow pattern (Fig. 22-4D).
6. Sample gate angle. Be sure to set the sample gate at an angle less than 60 degrees to the vessel. Patterns suggestive of rejection may be seen if the sample gate is at a wrong angle (i.e., >60 degrees).
7. Because of surgical technique and body habitus, the transplant may be placed in a vertical position, which affects the pulsed and color Doppler signal. One may not be able to examine this type of transplant with ultrasound.
If a patient presents with fever of unknown origin and no collections are found around the transplanted kidney, look at the native kidneys or other areas where abscesses may develop (see Chapter 11). Intrahepatic or perihepatic abscesses may occur. Occasionally, transplant recipients develop pancreatitis due to steroid overadministration.
SELECTED READING
Bruno S, Ferrari SK, Remuzzi G, et al. Doppler ultrasonography in posttransplant renal artery stenosis: a reliable tool for assessing effectiveness of revascularization? Transplantation 2003;76(1):147–153.
Jacques BC. Renal transplant surgery. In: Ultrasound of abdominal transplantation. Sidhu PS, Baxter GM, eds. New York: Thieme; 2002:23–26.
Little AF, Dodd GD III. Postoperative sonographic evaluation of the hepatic and renal transplant patient. Ultrasound Q 1995;13:111–119.
Pozinak MA. Doppler ultrasound evaluation of renal transplantation. In: Clinical Doppler ultrasound. London: Churchill Livingstone; 2000:191–201.
Patel U, Haw KK, Hughes NC. Doppler ultrasound for detection of renal transplant artery stenosis-threshold peak systolic velocity needs to be higher in a low-risk surveillance population. Clin Radiol 2003;58(10):772–777.
Radermacher J, Mengel M, Ellis S, et al. The renal arterial resistance index and renal allograft survival. N Engl J Med 2003;349(2):115–124.
![]()
SONOGRAM ABBREVIATIONS
CBD Common bile duct
Ha Hepatic artery
IVC Inferior vena cava
Pv Portal vein
KEY WORDS
Allograft. The newly transplanted liver.
Bacteremia. Blood stream infection.
Ischemia. Impaired blood supply.
Seroma. A fluid collection composed of blood products.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree