- •
Presence or absence of great vessels crossing as they course just above the heart.
- •
Relationship of the great vessels to each other as they arise from the heart.
- •
Relative size of the great vessels—is the pulmonary artery larger than the aorta by approximately 25%, as is expected in transposition of the great arteries?
- •
Presence or absence of ventricular septal defects.
- •
Presence or absence of left or right outflow tract obstruction.
- •
Presence or absence of semilunar valve stenosis.
- •
Presence or absence of a patent ductus arteriosus with shunting from the pulmonary artery to the descending aorta.
- •
Ductus arteriosus is typically smaller than in normally related great vessels.
- •
Ductal and aortic arches appear as running parallel in their course in the superior mediastinum.
- •
Atrial septal defect and redundancy of the septum primum.
Anatomy and Anatomical Associations
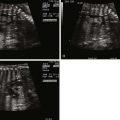
Transposition of the great arteries (TGA) is a congenital anomaly in which the aorta arises from the right ventricle and the pulmonary artery from the left ventricle, creating discordance in the ventricular-arterial relationship. In the normal heart, the aorta is posterior and rightward arising from the left ventricle and the pulmonary artery sits anterior and leftward arising from the right ventricle. In the most common form of TGA, the aorta is anterior and rightward with concordant atrioventricular relationships and normal d -looping of the ventricles. The conus is subaortic in TGA, as opposed to subpulmonic in normal, and there is fibrous continuity of the mitral and pulmonary valves in TGA, unlike fibrous continuity between the mitral and the aortic valves in the normal ( Figure 14-1 ). This common form of transposition is referred to as d -TGA and is the focus of this chapter. TGA can occur in more complex settings such as single-ventricle lesions, heterotaxy, and l -looped hearts. These complex forms of TGA are discussed in their respective chapters.

Associated lesions are common in fetuses with d -TGA and are important to identify in order to reliably predict postnatal stability and suitability for specific surgical strategies after birth. Approximately 50% of fetuses with d -TGA will have an intact ventricular septum and no associated cardiac defect other than a patent foramen ovale and a patent ductus arteriosus.
Frequency, Genetics, and Development
The incidence of d -TGA is approximately 2.5 per 10,000 live births and remains one of the more common causes of cyanotic congenital heart disease (CHD). There is a strong male predominance that accounts for 60% to 70% of live d -TGA births. At this time, there is no known causative genetic abnormality associated with d -TGA Most frequently, there is a normal karyotype and extracardiac abnormalities are relatively unusual in this form of CHD.
The developmental abnormalities that lead to d -TGA remain unknown. One of the more common theories relates to abnormal embryonic development of the normal anterior and leftward subpulmonary conus. This theory suggests that abnormal resorption of the subpulmonary conus with abnormal persistence of subaortic conus leads to the migration of the great vessels to their abnormal, discordant locations. A second theory focuses on abnormal spiraling of the primitive truncus arteriosus as the cause of a rightward and anterior aorta originating from the right ventricle.
Prenatal Physiology
The hemodynamic alterations in the fetus with d -TGA are tolerated well and are compatible with excellent fetal survival rates. There is usually normal growth and development with the fetus reaching term and of generous birth weight. In the fetus with d -TGA, the patency of the foramen ovale and the ductus arteriosus allows for mixing of circulations and maintenance of adequate oxygenation and a normal cardiac output. Nonetheless, there are alterations in blood flow that have the potential to affect the fetus with d -TGA. Normally, highly oxygenated blood in the umbilical vein has preferential return from the ductus venosus directed across the foramen ovale into the left side of the heart. The left ventricle and pulmonary arteries receive blood with a relatively high oxygen concentration. As well, deoxygenated blood from the superior vena cava is preferentially directed across the tricuspid valve to the right ventricle. This pattern is a natural strategy deemed to provide the most vital organs that are normally perfused by the left ventricle—the heart and brain—with the greatest degree of oxygenated blood. In the normal fetus, the right ventricle ejects into the descending aorta via the ductus arteriosus. In d -TGA, the right ventricle ejects into the ascending aorta, supplying blood with a lower oxygen concentration to the head and neck vessels compared with a fetus with normal ventricular-arterial relationships. Animal studies in fetal lambs have demonstrated a partial pressure of oxygen (PO 2 ) of approximately 18 mm Hg in the ascending aorta in d -TGA, which is significantly less than the values of 25 to 28 mm Hg seen in normal fetuses. The full extent of these alterations is not fully understood in the human fetus; however, it is safe to assume that cerebral oxygenation in the human fetus with TGA may not be normal.
Prenatal Management
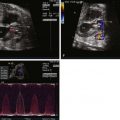
TGA is commonly missed on prenatal scanning. The four-chamber view will appear normal and the diagnosis of this critical heart defect requires inspection of the great vessels arising from the heart. Training and experience of obstetricians and perinatologists who perform imaging will increase the diagnosis and improve outcome. Normally related great vessels will cross in space as scanning of the heart moves cephalad and superiorly—the larger pulmonary artery will be seen arising from the right ventricle and crossing over the more posterior aorta arising from the left ventricle. The appreciation of this relationship takes time; however, it should be performed in every fetus that presents for evaluation. In d -TGA, the great vessels arise from the heart in parallel and do not cross.
When the diagnosis of d -TGA is made in a fetus, it is important to identify the presence of associated intracardiac anomalies. Similar to other forms of CHD, there is an indication for analysis of the fetal chromosomes and an obstetrical ultrasound to evaluate for extracardiac anomalies. This allows the caretaker to accurately counsel families in regard to potential suitability for an arterial switch operation, the optimal postnatal treatment, and provide information regarding outcomes and survival rates. It is important to mention the potential need for postnatal balloon atrial septostomy (BAS), which is necessary in approximately 20% to 40% of neonates with d -TGA. This procedure is done in order to improve the mixing of oxygenated and deoxygenated blood at the level of the atria and improve overall arterial saturation. At our center, we serially evaluate the fetus with d -TGA every 4 weeks to monitor for restriction at the foramen ovale and ductus arteriosus. This also allows for surveillance of ventricular function and fetal growth and to document progression of any associated defects such as pulmonary stenosis or coarctation of the aorta.
The status of the foramen ovale and the ductus arteriosus are two key structures that predict postnatal stability. Restriction at either structure will limit mixing between the circulations and put the fetus at risk for hemodynamic compromise and severe hypoxemia after separation from the placenta. Careful evaluation of the atrial septum and ductus arteriosus should be performed in every fetus with d -TGA.
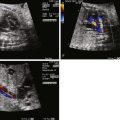
Predicting the need for urgent postnatal BAS based on prenatal data, however, is very challenging. In the fetus, the flap of the foramen ovale will almost always bow right to left into the left atrium; however, with birth and an increase in pulmonary venous return, left atrial pressure rises and the flap of the foramen ovale may close, causing impaired atrial level mixing and severe hypoxemia. In order to attempt to predict postnatal atrial level restriction based on prenatal atrial findings, we have developed an index that describes the degree of redundancy of the atrial septum, with the theory being that the greater the atrial redundancy, the less likely it will become restrictive once left atrial pressures rise after birth. The atrial septal excursion ratio (ASE ratio) is obtained in the four-chamber view. It is a measure of the ratio of the maximum excursion of the flap of the foramen ovale leftward toward the free wall of the left atrium from the anchored portion of the septum divided by the diameter of the left atrium from septum to free wall ( Figure 14-2 ). The greater this ratio, the less likely there should be a need for urgent BAS. In a recent review, we found that no fetus with a ratio greater than 0.5 required urgent BAS at birth (unpublished data).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree