Major risk factors for hemorrhage
Annual bleeding risk (%)
1. Previous ICH
4.5
2. Deep location
3.1
3. Exclusively deep venous drainage
2.5
Other risk factors often considered to predict future hemorrhage:
Stenotic/occlusive changes in the draining vein
Single draining vein
Intranidal aneurysms
Small nidal size
Some estimate risk the lifetime of bleeding by subtracting age from 105.
Contraindications
Difficult access – including tortuous/occluded proximal arterial anatomy
Iodinated contrast allergy
Severe renal impairment
Anatomy
Thorough angiographic assessment should consider each of the following:
Arteries
Type and number of feeding arteries
Aneurysms (remote, feeding vessel, or intranidal)
Induced collaterals from adjacent vascular territories (may be misinterpreted as part of the nidus)
Shunt
Nidus compact or diffuse
Presence of fistula – fast/slow?
Veins
Numbers – deep/superficial
Stenosis/ectasia
Degree of congestion
Spetzler-Martin Grading (Grades I–V) (Table 2 and 3)
Table 2
Spetzler-Martin grade
A point is allocated for size, location, deep venous drainage | |
Eloquent cortex is sensorimotor, language, visual cortex, hypothalamus, internal capsule, brainstem, cerebellar peduncles, and deep cerebellar nuclei | |
A separate SM grade VI is considered inoperable | |
Size | Point |
Small (<3 cm) | 1 |
Medium 3–6 cm | 2 |
Large >6 cm | 3 |
(Usually at the time of diagnosis, 30 % is <3 cm, 60 % 3–6 cm, and 10 % >6 cm). | |
Location | |
Non-eloquent | 0 |
Eloquent | 1 |
Venous drainage | |
Only superficial | 0 |
Deep (any) | 1 |
Table 3
Incidence of postoperative deficit by AVM SM grade
Minor | Major | |
|---|---|---|
I | 0 | 0 |
II | 5 | 0 |
III | 12 | 4 |
IV | 20 | 7 |
V | 10 | 12 |
Widely used – primarily reflects surgical risk but broadly reflects embolization; risk large complex AVMs require multiple procedures
Higher risk with those related to eloquent cortex, deep feeders, and deep venous drainage
BUT does NOT consider perforator arterial supply, compact on diffuse nidus, associated aneurysms, and, importantly, the experience of the neurosurgeon/interventionalist
Equipment
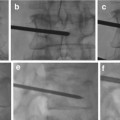
Biplane angiography is considered essential.
6-F guide catheter with heparinized saline flush.
Microcatheter: DMSO compatible for Onyx usage.
Microwire.
Embolic Agents
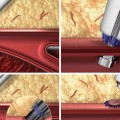
Liquid embolic agents – penetrate deeply into the AVM achieving permanent embolization – n-butyl cyanoacrylate (NBCA) and Onyx
Detachable coils – may be helpful adjunct to slow flow in fistulous components
Particles generally ineffective – high rate of recanalization
NBCA/Histoacryl/“Glue”
NBCA is a liquid monomer that undergoes a rapid exothermic polymerization catalyzed by nucleophiles found in blood and on the vascular endothelium, to form a solid adhesive.
NBCA provokes an inflammatory response in the vessel wall and surrounding tissue, leading to vessel necrosis and fibrous ingrowth.
Recanalization is very uncommon after an adequate embolization.
The rate of polymerization is adjusted by diluting NBCA with lipiodol, which is also radiopaque, and allows visualization during injection.
Higher concentrations of lipiodol will reduce the rate of polymerization and increase the viscosity of the embolic material. Dilute NBCA penetrates deeper into the nidus but with greater risk of escape into the venous system.
Tantalum powder may be added to provide greater radiopacity and is essential where very high concentrations of glue (>90 %) are required.
Positive: NBCA may be preferred to Onyx in some fistulous AV shunts, perforating arteries, leptomeningeal collaterals, en passant feeders, and when the catheter position is away from the nidus.
Negative: NBCA is generally considered less predictable than Onyx, even in experienced hands.
Onyx
Onyx is ethylene vinyl copolymer (EVOH) dissolved in dimethyl sulfoxide (DMSO) and made radiopaque with tantalum powder.
A nonadhesive, cohesive liquid – on contact with blood, the DMSO solvent rapidly diffuses away causing precipitation and solidification of the polymer, a permanent spongy material (complete within 5 min).
Solidification is slower than with NBCA, allowing prolonged controlled injections.
Precipitation progresses from the outer surface inward, forming a skin with a liquid center that continues to flow (like lava) as the solidification continues.
Rate of precipitation of copolymer is proportional to concentration of EVOH.
Onyx 18 (6 % EVOH) is less viscous (viscosity 18 cP), will generally flow further from the catheter tip, and is often used for embolization of the plexiform nidus.
Onyx 34 (8 % EVOH) is more viscous (viscosity 30 cP) and is generally used for higher flow fistulas.
Positive
Better nidal penetration from a single pedicle. Multidirectional nidus penetration with retrograde filling of other feeders.
Control angiography possible
mid-injection to assess nidus and draining veins.
More pliable/less inflammatory than NBCA – easier surgical manipulation.
Negative
DMSO toxic effects include vasospasm, angionecrosis, arterial thrombosis, and vascular rupture. Toxicity directly related to the volume infused and endothelial contact time. To avoid angiotoxicity: Use SLOW DMSO infusion rate not exceeding 0.25 ml/90 s.
Limited to DMSO-compatible microcatheters/syringes, etc.
Tantalum may cause sparking with bipolar cautery during surgery.
Artifact on CT but not MRI (hypointense on T1- and T2-weighted sequences).
Pre-procedure Medications
The author uses dexamethasone 12 mg intravenously at the beginning of each procedure to limit any inflammatory response that might be induced by the embolic agent.
The Procedure
Access
Transfemoral catheterization of the parent vessel (internal carotid, vertebral, or occasionally external carotid artery) with a 6 F guide catheter with continuous heparinized saline flush.
If the AVM is supplied by >2 vascular territories, e.g., posterior and middle cerebral, the author places guide catheters in both parent vessels (vertebral and ICA) to control the whole arterial territory during embolization.
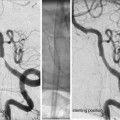
Angiography and Assessing the Lesion
A minimum of four-vessel angiography should be repeated at the start of each session of embolization. External carotid artery injections should be considered wherever dural supply is possible – superficial lesion/previous intervention.
Provocative Testing
Involves the selective injection of a short-acting anesthetic into the territory in question in the awake patient followed by neurological testing of that region – amobarbital and methohexital are injected into brain arteries and lignocaine to test cranial nerves in external carotid branches.
Why? Absence of visible normal vessels on superselective angiography does not guarantee embolization without deficit.
A negative result is not 100 % predictive – technique not in widespread usage.
Treatment Plan and Technique
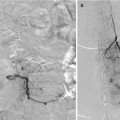
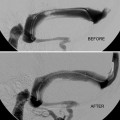
Ultimate goal is usually cure/obliteration – often requiring multimodality approach.
Any residual early venous filling risks subsequent ICH.
Partial treatment may increase hemorrhagic risk.
Invasive treatment in unruptured AVMs may be more risky than natural history (a Randomized Trial of Treatment of Unruptured Brain AVMs (ARUBA) attempts to address this).
Management requires a careful, experienced multidisciplinary approach.
Treatment options include conservative management, surgery, stereotactic radiosurgery (STRS), and embolization in any combination.
No randomized trials have compared treatment types with one another or with natural history.
Consider
Patient factors: presentation, age, comorbidity, occupation, and lifestyle
AVM factors: location, size, and angioarchitecture
Technical factors: local experience/expertise
Embolization Strategies
There must be a clear and realistic embolization strategy from the outset.