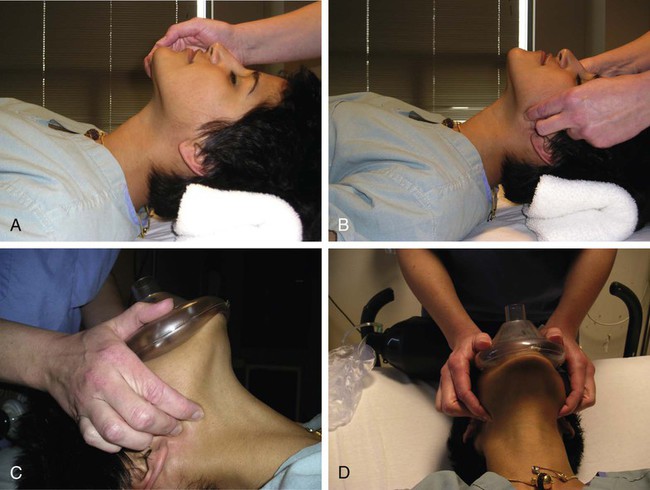
Procedural sedation/analgesia is a common and important component of interventional procedures. Appropriate patient positioning and maintaining patient comfort are facilitated by proper sedation. However, reaching the perfect balance of sedation and comfort without oversedating the patient can be challenging. Striking the right balance can be particularly difficult in patients with underlying lung disease and in patients with unpredictable metabolism of common sedatives. In addition, patients with a marginal circulatory status may be prone to hemodynamic effects. The oversedated patient can experience hypercarbia, hypoxemia, and/or hypotension either related to the sedative itself or due to secondary effects. Prevention of oversedation and hypercarbia can be prevented with close patient monitoring and the use of pulse oximetry; the patient’s response to verbal commands; observation of ventilation, blood pressure (BP), and heart rate every 5 minutes; electrocardiography for patients with significant cardiovascular disease; and use of exhaled capnography. When patients become oversedated, it is common for minute ventilation to decrease, which is often associated with a rise in end-tidal CO2 (ETCO2). This elevation typically precedes apnea and can alert the physician that the level of sedation is excessive. ETCO2 above 50 mmHg, an absolute change of over 10 mmHg, or an absent waveform may detect subclinical respiratory depression not detected by pulse oximetry alone.1 This is vitally important, since many patients have a variable response to hypnotic drugs. The choice of drugs that have minimal hemodynamic and respiratory depression may be considered (e.g., etomidate,2 ketamine,3 ketamine-propofol combination4). • Use of narcotics can cause vomiting and aspiration. • Narcotic and benzodiazepine use decreases respiratory drive and can cause central apnea. • Muscle relaxation caused by sedatives can cause upper airway obstruction. The approach to the oversedated patient should be managed in a more airway-centered manner. Once oversedation is recognized, a staff member in the interventional suite should be detailed to the head of the bed to manage the airway. Noninvasive airway management should be sufficient in most cases. If stimulation of the patient (e.g., calling the patient’s name loudly, sternal rub) fails to immediately correct the compromise in oxygenation or respiratory effort, the airway should be assessed and optimized (Fig. 16-1; also see Airway Management and Optimization, later). At this point, if the sedative used has an antidote (e.g., flumazenil for benzodiazepines or, naloxone for narcotics), that antidote should be drawn up (Table 16-1). TABLE 16-1 Sedative Antidote Administration Optimization of the airway often requires repositioning the patient’s head and neck. A simple understanding of the anatomy allows anyone to place the patient’s head in more favorable position for gas exchange. The tongue is the most common cause of upper airway obstruction. A head-tilt/chin-lift or jaw-thrust maneuver (Fig. 16-2) also called the sniffing position may establish an airway. If proper positioning fails to establish a viable airway, however, more invasive maneuvers such as using a nasopharyngeal/oropharyngeal airway or a laryngeal mask airway (LMA) may be necessary and will be discussed in a later section.5 Both benzodiazepines and narcotics have reversal agents that if used correctly could save an oversedated patient from having a respiratory arrest. Flumazenil antagonizes the action of benzodiazepines on the central nervous system and inhibits activity at γ-aminobutyric acid/benzodiazepine receptor sites. It is contraindicated in patients with serious tricyclic overdoses.6 Available in injectable 0.1 mg/mL, flumazenil can be given undiluted or in D5W, lactated Ringer’s solution, or 0.9 normal saline. The initial intravenous (IV) dose is 0.2 to 0.4 mg over 15 seconds. If ineffective, a repeat dose can be given every 60 seconds. Maximum initial dose is 1 mg. If the patient becomes oversedated, a repeat dose can be given, and a drip may have to be started if the half-life of the offending benzodiazepine is longer than the half-life of flumazenil (41-79 minutes). Adverse effects include seizures, dizziness, headache, blurred vision, diplopia, visual field deficit, hyperventilation, nausea, and vomiting.6 Naloxone hydrochloride is a pure narcotic antagonist. It reverses the respiratory depression, sedation, and hypotensive effects of opioids. In the absence of narcotics, naloxone has no activity. Caution must be used in narcotic addiction, cardiac disease, and use of cardiotoxic drugs. Rapid reversal of narcotic depression may also cause nausea, vomiting, diaphoresis, and circulatory stress. The initial dose is 0.1 to 2 mg IV at 2- to 3-minute intervals until the desired effect of reversal is reached. It can be given undiluted as a bolus or as an IV infusion with D5W or 0.9 normal saline. As with flumazenil, a drip may have to be started owing to a longer half-life of the offending agent. Naloxone has a half-life of 30 to 81 minutes. As an infusion, use 3.7 µg/kg/h. Adverse effects include seizures, ventricular tachycardia, ventricular fibrillation, acute narcotic abstinence syndrome, and return of pain.7 If stimulation, airway optimization, and reversal agents fail to rectify the situation, airway compromise may be the underlying problem, in which case the clinician should continue down the airway management algorithm (see Fig. 16-1).
Treatment of Medical Emergencies
Oversedation
Antidote
Dosage and Comments
Side Effects
Naloxone
0.4-2 mg at a time; maximum dose 10 mg
For typical doses used for conscious sedation, a dose of 0.2-0.4 mg should be sufficient
Can be given as an infusion (2 mg/100 mL) 0.1 mg/min7
As narcotic reverses, elevated blood pressure, tachycardia, nausea, and vomiting can occur
Flumazenil
0.2-1 mg dose
Each 0.2-mg increment should be given over 1 minute
In clinical trials, 75% of patients responded to a dose of 1-3 mg
Frequent side effects (≈10%): nausea, vomiting, headache, blurred vision
Major side effect (<1%): seizure6
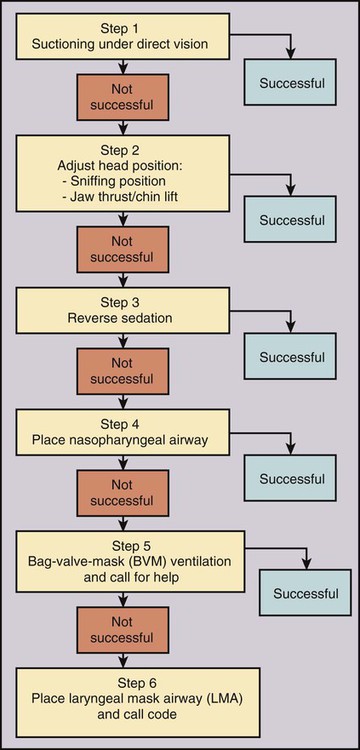
Treatment of Medical Emergencies