and Ronit Agid2
(1)
Department of Medical Imaging, Division of Neuroradiology, Toronto Western Hospital, Toronto, ON, Canada
(2)
Department of Medical Imaging, Division of Neuroradiology, Toronto Western Hospital, University of Toronto, Toronto, ON, Canada
Abstract
Historically, craniofacial lesions have been treated surgically. The advent of image-guided endovascular techniques, however, enabled their application to the treatment of vascular malformations and tumors. Optimal treatment requires a multidisciplinary approach. This will be center specific and determined by available expertise and skills. Goals of treatment, nature of the lesion, and its associated angioarchitecture influence decisions regarding treatment planning. Venolymphatic malformations are typically treated by percutaneous sclerotherapy, with or without surgical adjunct. Different sclerosants are described in the literature, with bleomycin increasingly chosen because of its efficacy and favorable side effect.
Keywords
Percutaneous venolymphatic malformationsExtracranial AV malformationsAlcoholSclerosantsExtracranial arteriovenous malformationsFacial AVMsIntroduction
Historically, craniofacial lesions have been treated surgically. The advent of image-guided endovascular techniques, however, enabled their application to the treatment of vascular malformations and tumors.
Optimal treatment requires a multidisciplinary approach. This will be center specific and determined by available expertise and skills.
Goals of treatment, nature of the lesion, and its associated angioarchitecture influence decisions regarding treatment planning.
Venolymphatic malformations (VLMs) are typically treated by percutaneous sclerotherapy, with or without surgical adjunct (Fig. 1). Different sclerosants are described in the literature, with bleomycin increasingly chosen because of its efficacy and favorable side-effect profile.
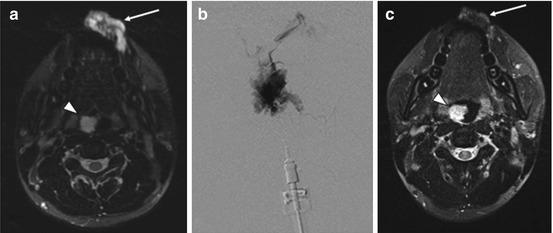
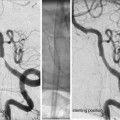
Fig. 1
Percutaneous sclerotherapy of facial venolymphatic malformation. A 25-year-old man presented with a left lower lip vascular malformation. Axial T2 fat sat MRI demonstrated a hyperintense lesion, consistent with a VLM (arrow a). Percutaneous sclerotherapy was performed using bleomycin. Throughout the procedure, each needle insertion was followed by phlebography to confirm location within the lesion and assess drainage (b). This was followed by injection of bleomycin under continuous subtraction fluoroscopy using the negative subtraction technique. A total of 3 sclerotherapy sessions was completed. Follow-up MRI performed several months after treatment demonstrates a significant reduction in size with a small area of residual hyperintensity (arrow c). This particular patient had multiple craniofacial VLMs, including one in the oropharynx (arrow head a, c), which was later treated with airway protection. MRI magnetic resonance imaging, VLM venolymphatic malformation
Extracranial arteriovenous malformations (facial AVMs) are generally treated by preoperative embolization, followed by surgical resection. Typically, this is done transarterially, with percutaneous injection of embolic agents sometimes used as an adjunct (Figs. 2 and 3).
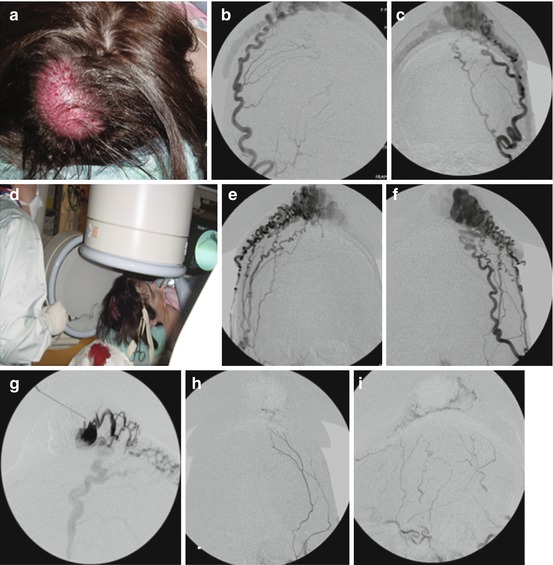
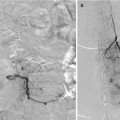
Fig. 2
Percutaneous embolization of scalp AVM with NBCA. Young women presented with progressive enlargement of a scalp AVM (a). Diagnostic angiogram showed bilateral supply this AVM from the external carotid arteries (b, c, e, f). AP views of right (b) and left (c) occipital artery and right (e) and left (f) distal external carotid artery are shown. These arteries were extremely torturous and it was unlikely to obtain an optimal distal positioning of microcatheter to cure the AVM via transarterial embolization. A tourniquet was applied to the patient’s head around the nidus of the lesion and a butterfly needle was inserted into the nidus (d). Diagnostic phlebography was performed through each direct puncture prior to injection of glue (g). This was followed by percutaneous glue embolization which resulted in cure of AVM. Post-embolization angiogram of the left external carotid artery in AP (h) and lateral (i) projection shows no residual shunting. NBCA n-butyl cyanoacrylate, AVM arteriovenous malformation
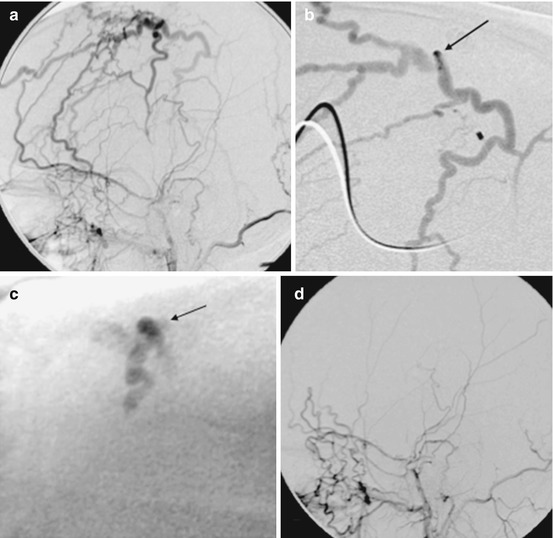
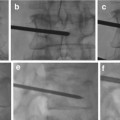
Fig. 3
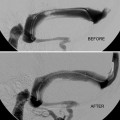
Percutaneous embolization of iatrogenic AVF of the scalp. This fistula developed following hair transplantation. (a) Lateral angiogram of right ECA injection shows a single whole AVF supplied by tortuous branches of the superficial temporal artery and drains into several scalp veins. (b) Selective angiogram via direct percutaneous needle at the fistulous site showing the draining veins (arrows shows puncture site). (c) Image of the glue cast after embolization with NBCA through the percutaneous needle (arrow shows the puncture site). (d) Post-embolization angiogram of ECA shows complete cure of the AVF. AVF arteriovenous fistula, NBCA n-butyl cyanoacrylate, ECA external carotid artery
Because of their hypervascularity, embolization of craniofacial tumors has become an established technique, either for curative, preoperative, or palliative management. Initially, transarterial approaches involving a variety of embolic agents were described. (Percutaneous treatment, alone or in conjunction with endovascular embolization, is increasingly preferred because of easier access and, as a result, more complete devascularization.)
Using Embolic and Sclerosing Agents in the Treatment of Head and Neck Lesions
Selection of Approach and Choice of Embolic Agent
Decisions regarding approach are contingent on the type of lesion and management goals. Definitive treatment will require a different modality than temporary diminishment of vascular supply (i.e., as for presurgical devascularization). This will inform the decision to use a liquid embolic, particles, or a sclerosant.
Embolization is the intentional occlusion of vasculature using a foreign material (e.g., n-butyl cyanoacrylate (NBCA), ethylene vinyl alcohol copolymer (onyx), or polyvinyl alcohol (PVA) particles). Sclerotherapy, on the other hand, is the obliteration of a blood vessel using an agent (e.g., alcohol, bleomycin) that causes endothelial cell destruction and thrombosis through denaturation and cellular dehydration. There is no current consensus in the literature as to which agent/technique is ideal for a given clinical situation, with center-specific preferences and local expertise largely determining practice.
Prior to selecting an embolic agent, it is important to consider why the embolization is being performed, whether temporary or permanent occlusion is required, the diameter of the vessel to be embolized, what is downstream from it, and the velocity of flow through this vessel.
Whether performing percutaneous embolization or sclerotherapy, phlebography should always be completed as an initial step prior to treatment. This is important for complete visualization of the angioarchitecture of the lesion as well as its venous drainage.
Liquid Embolics
These include the cyanoacrylate glues, most commonly NBCA and onyx. They are preferred clinically because of their low recanalization rates and are thus appropriate for permanent vessel occlusion (e.g., as in treatment of AVMs).
Cyanoacrylate, or N–butyl-2-cyanoacrylate (NBCA), is a rapidly hardening liquid adhesive that polymerizes immediately on contact with blood or other ionic fluids. Polymerization causes an exothermic reaction that destroys the vessel wall.
Because it hardens so quickly, controlling its final position can be difficult. As a result, safe use of this material requires extensive training and experience.
Onyx is an ethylene vinyl alcohol copolymer dissolved in dimethyl sulfoxide (DMSO), which uses micronized tantalum as a contrast agent. When injected into an AVM, the DMSO dissipates, causing the copolymer to form a spongy occlusive cast in the artery. It propagates easily into vessels and has a consistency that makes it easier to resect than some cyanoacrylates used as embolic agents. Drawbacks include a need for slow injection, which results in a longer procedure with relatively increased radiation exposure for both patient and surgeon.
Injection of any liquid embolic may result in opening of unexpected anastomoses and is thus associated with a risk of cerebral infarction, cranial nerve palsy, and cerebral hemorrhage.
Particles
These are available in variable sizes (50–2,000 μm) and are relatively inexpensive. They cause a mild inflammatory response and nonpermanent occlusion, thus making them appropriate only for temporary embolotherapy. Typically, they are used for preoperative devascularization of tumors. They are also used to induce thrombosis in a vessel secondary to partial success of an earlier endovascular embolization procedure. Drawbacks include recanalization, poor radiopacity, and the possibility of embolization to the arteries of lungs, brain, or cranial nerves.
Sclerosants
All sclerosants work by causing damage to vascular endothelia through various mechanisms.
Ethanol (absolute alcohol) is the most commonly used sclerosant. It has a direct toxic effect on vascular endothelia that activates the coagulation system and causes microaggregation of red blood cells. It damages capillary beds of healthy tissue (e.g., skin) and is usually associated with significant soft tissue swelling and pain on injection. As such, it is usually administered under general anesthesia.
Most other commonly used sclerosants are alcohol derivatives, e.g., sodium tetradecyl sulfate (Sotradecol) and ethanolamine oleate. Use of these agents is both less painful and toxic then absolute alcohol. As a result, some lesions can be treated without general anesthesia.
Recently, the off-label use of bleomycin – a cytotoxic antitumor agent – has been described. Its sclerosing effect on endothelial cells was originally applied to the treatment of venous malformations and hemangiomas. Since this initial report, a number of studies have been published, supporting both the treatment efficacy and relatively low complication rate.
Regarding possible complications of sclerosants and safety measures (see chapter “Diagnostic Cerebral Angiography and Groin Access and Closure”).
Delivery: Endovascular Versus Percutaneous
Transarterial embolization is an established technique to devascularize lesions; results may be curative, preoperative, or palliative. However, endovascular embolization may be limited due to vessel tortuosity, vasospasm, atherosclerotic disease, or very small arterial feeders.
Because of these limitations, there has been increasing use of percutaneous embolization (PTE) to devascularize head and neck lesions. Initially, this was applied to the treatment of VLMs (Figs. 1 and 2). More recently, this approach has been applied to the treatment of craniofacial AVMs (Figs. 2 and 3) and craniofacial tumors.
PTE has allowed easy access to the lesion and overcomes the limitations of endovascular treatment. Though still an emerging modality, some authors have stated a preference for it, believing that PTE allows for a more complete devascularization of craniofacial lesions.
Indications for Intervention
Venolymphatic Malformations
Pain, functional impairment (secondary to swelling or pain), or esthetic concerns.
Absolute indications include lesions that threaten vital functions because of their location and mass effect (i.e., vision, airway compromise).
Facial AVMs
Hemorrhagic events, pain, and ulceration.
Treatment is absolutely indicated in cases associated with symptomatic congestive heart failure and where vital functions are threatened.
Less commonly it may be provided for issues of cosmesis.
Asymptomatic AVM should be observed unless possible to cure with minimal morbidity; embolization or incomplete excision may stimulate regrowth and increased symptoms.
Tumors of Head and Neck
Preoperative embolization to reduce perioperative blood loss.
Palliation of symptoms related to a growing tumor, including compression and destruction of adjacent structures.
Contraindications to Intervention
Absolute
Known anaphylactic reaction to contrast or the intended embolic agent
Relative
Inability to tolerate a general anesthetic.
Kidney failure, which can be treated with prophylactic intravenous hydration and bicarbonate therapy plus oral dosing of N-acetyl cysteine.
A high degree of risk associated with inherent post-procedural swelling due to lesion location, e.g., airway compromise. Swelling can be partially prevented by steroid administration at the end of the procedure and for several days thereafter. In extreme cases patients may require prophylactic tracheostomy prior to the procedure.
General Patient Preparation
General anesthesia or conscious sedation is commonly used, although the decision of whether or not they are employed and the choice of one over the other are center specific.
The procedure is performed under sterile conditions in an imaging suite that is adequately equipped for the modality chosen. The patient is positioned and draped in a sterile fashion that provides best access to the lesion.
Percutaneous Treatment of Venolymphatic Malformations of the Head and Neck
Clinical Features
Venolymphatic malformations (VLMs) are rare with a prevalence of 1 % and an estimated incidence of 1–2 in 10,000 births. They represent, however, a common reason for referral to interventional radiology departments in vascular malformation centers.
These congenital anomalies consist of “slow-flow” dysmorphic channels lined by flattened endothelium and a thin basement membrane.
In VMs these channels are filled with venous blood and, in LMs, with lymphatic fluid.
Historically, there have been issues with misdiagnosis and classification. In 1982 Mulliken and Glowacki developed a dichotomous classification system to which several subdivisions have since been proposed. The most clinically relevant does so on the basis of vascular dynamics, with arteriovenous malformations (AVMs) classified as high flow and venous malformations (VMs) as low flow. Although lymphatic malformations (LMs) are considered separately, most clinicians regard them as fitting in the same category as VMs because of both similar flow characteristics and treatment.
Though present at birth, venolymphatic malformations (VLMs) may become clinically apparent at any time throughout the lifespan.
Diagnostic Evaluation
VMs present in various ways, from a vague blue patch on the skin to a soft, compressible, nonpulsatile mass; 40 % of cases are found in the head and neck region.
The most common symptoms include pain, compression of adjacent structures, and cosmetic deformity.
Compressive symptoms relate to location of the lesion and compression of adjacent structures. In the oropharynx, they may cause dysphagia and airway compromise. Lesions located in and around the orbit may result in visual issues and impaired extraocular movements.
Physical exam typically demonstrates a soft, compressible, blue-tinged mass that enlarges with dependent positioning or Valsalva.
Confirmation of diagnosis with imaging is indicated in order to rule out other entities such as malignant solid tumors of the head and neck.
Magnetic resonance (MR) imaging is largely recognized as the gold standard. The most useful sequence is fat saturated T2 weighting (Fig. 1). The classic MR appearance of VMs is that of a soft tissue mass that is hyperintense on T2-weighted images and hypointense on T1-weighted images. Multiple, rounded hypointense areas on T2 representing phleboliths, or intralesional calcifications, may be noted. Enhancement after intravenous (IV) administration of gadolinium will be noted, mostly peripherally.
LMs have a similar appearance on MR, being hyperintense on T2 and hypointense on T1. These non-enhancing lesions appear as cysts containing fluid-fluid levels; phleboliths are not seen.
Ultrasonography is frequently used as an initial imaging modality because of its low cost, widespread availability, and lack of ionizing radiation, a particular concern given the fact that lesions are often diagnosed in the pediatric population.
The primary utility of ultrasound is to differentiate between high-flow (AVM) and low-flow (VM, LM) lesions and, if the latter, to distinguish LMs from VMs based on their cystic appearance.
Treatment
Conservative management is advocated for those lesions that do not meet the threshold for intervention. It consists of reassurance, elevation of the involved area during sleep, and avoidance of activities associated with symptom exacerbation.
Cessation of oral contraceptives may provide relief for those whose lesions are hormonally sensitive.
When indicated, the treatment of choice is percutaneous sclerotherapy with or without surgical excision.
Laser therapy has also been described in the literature.
Multidisciplinary approaches are emphasized, such that combinations of sclerotherapy, surgery, and laser can be tailored to patient preferences and lesion anatomy.
Patient Preparation
Patient’s expectations regarding treatment outcome should be clearly established. Specifically, the patient should understand that sclerotherapy does not completely cure VLMs but rather leads to decrease in size. Clinical benefit is seen in 75–100 %, dependent on both the definition of response and sclerosant used.
Patients should be warned about the post-procedural swelling inherent to treatment, particularly if alcohol is to be used as a sclerosant.
Patients with tongue or intra-orbital lesions are more prone to post-procedural swelling and infection. Thus, they should be prophylactically treated with steroids and antibiotics for several days after the procedure. Strong consideration should also be given to the choice of bleomycin as sclerosant, as it is associated with less post-procedural swelling.
Treatment Techniques
Direct Percutaneous Sclerotherapy
Sclerotherapy begins with the introduction of a needle into the lesion, whose location is usually apparent by palpation. Alternatively, US can be used for guidance.
The needle is advanced until blood (for VMs) or lymphatic fluid (for LMs) is noted in the hub. This signifies intraluminal positioning of the needle.
Small amount (1–2 cc) of low-osmolarity, water-soluble iodinated contrast material is injected for phlebography, which should be performed prior to sclerotherapy.
Phlebography verifies needle location and provides information regarding flow characteristics and drainage of the lesion. This is important to ensure there is no outflow to critical structures (e.g., cavernous sinus) and is of particular importance when using alcohol.
If the VLM drains to risky areas, these should be blocked by external compression and phlebography repeated to ensure its effectiveness.
Slow injection of sclerosant is then performed under digital subtraction angiography at a rate of one image every 2 s. The sclerosant injected into the lesion gradually replaces the previously injected contrast, pushing it towards the periphery of the lesion or into the draining veins of the VM. It appears as negative contrast (white) on DSA (Figs. 1 and 4).
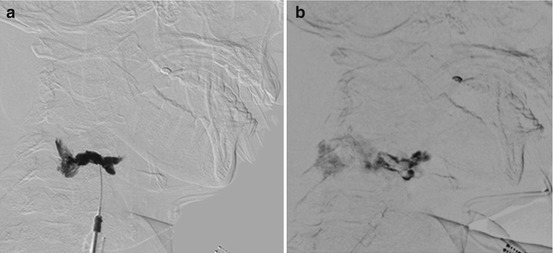
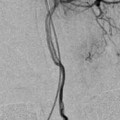
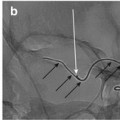
Fig. 4
Negative subtraction technique for sclerotherapy of VLM in the right neck: (a) RAO view of phlebography prior to sclerotherapy – contrast was injected into one of the pockets of the VLM. (b) Bleomycin injection under DSA into the region previously injected with contrast. Unopacified bleomycin is seen as a central white shadow due to “negative subtraction,” while the previously injected contrast is pushed to the periphery of the lesion and posteriorly towards other parts of the VLM in the neck. VLM venolymphatic malformations
This “negative subtraction” technique (Fig. 4) eliminates the need to premix the sclerosant with a contrast agent. As well, it allows real-time visualization of the sclerosant as it is injected into the VLM, while preserving its high concentration and potency as a sclerosing agent.
During injection of the sclerosant, the patient should be carefully monitored to assess for extravasation. Signs that may indicate chemical toxicity or ischemia to the skin include overly rapid efflux of sclerosant into the venous outflow, resistance to injection, or skin blanching. The presence of any of these should prompt immediate cessation of injection.
Typically 1–3 cc of sclerosant will be injected to each “pocket” of the malformation.
The procedure is repeated using multiple needle insertions, each followed by phlebography and sclerosant injection. This continues until the maximal dose is administered or when no blood return is noted, indicating that the VLM “pocket” is adequately treated.
According to our experience a total of 10 cc of alcohol per session or up to 15 mg of bleomycin per session is the recommended dose.
After the final injection all needles are removed. Light compression should be applied for several minutes until oozing at the puncture site stops.
Choice of Sclerosant
There is no consensus as to choice of sclerosant, with no randomized trials available to date. Currently, clinician familiarity, availability of the agent and lesion morphology has the greatest influence.
Alcohol (100 % ethyl alcohol) is most commonly used, owing to the fact that it is readily available, inexpensive, and effective. It is also associated with the highest rates of complications, including skin necrosis, nerve damage, and severe post-procedural swelling.
Other alcohol-based sclerosants include detergents and sclerosing foams. These are milder than alcohol and have lower complication rates. Concordantly, they also have lower potency and are less effective.
Aggressive sclerosants (e.g., alcohol), which cause significant post-procedural swelling, are not recommended for use on lesions proximal to the airway or orbit.
As it produces only minimal swelling after sclerotherapy (as opposed to ethanol-based agents), bleomycin is especially recommended for treatment of vulnerable areas such as the periorbit or regions related to the airway (i.e., the tongue and parapharyngeal spaces).
Modifications to Sclerotherapy Technique
The above-described negative subtraction technique (Fig. 4) is an elegant way to visualize the injected sclerosant without the need to mix and dilute it with contrast material prior to injection. This increases sclerosant concentration and thereby its therapeutic efficacy.
The use of alcohol to sclerose the draining vein of the lesion prior to bleomycin instillation has been described. This increases the therapeutic dwell time of the bleomycin within the lesion and decreases the dose (and potential toxicity) of the alcohol.
In recent years, sclerosants have begun to be administered in foam form, a technique that maximally displaces blood from the treated vessel being treated, remains undiluted, and persists within the vessel so as to maximize its sclerosant effect. The method of foam production first described by Tessari has become the dominant method.
Puig et al. have described a double-needle technique where a second needle is positioned away from the initial sclerotherapy needle but still within the lesion. Sclerosant is injected through the first needle and, as intralesional pressure increases, the combination of contrast, blood, and sclerosant exits out of the second needle, following the path of least resistance. This technique allows for close monitoring of the path and sclerosant distribution of the sclerosant and avoids passage into the systemic circulation. Additionally, by dispersing intraluminal pressure, the second needle prevents extravasation into adjacent normal vessels and thus local tissue damage.
Post-procedure Management and Follow-Up
The pain and swelling associated with the post-sclerotherapy period almost invariably necessitates prescription of analgesics or anti-inflammatory agents.
Steroids are not routinely given, although stronger sclerosants, larger treatment areas, or proximity to vital structures may necessitate their use.
There is no consensus in the literature as to length of hospital stay post-procedure. Typically, the patient can be discharged after recovery from anesthesia.
Patients should be seen in follow-up 3–4 weeks subsequent to their procedure. This visit can be used to assess level of satisfaction and need for further therapy. Additional ultrasound examinations may also be performed to evaluate flow and regional involution to direct further therapy.
Most lesions require more than one session of treatment. Should the lesion require additional sclerotherapy sessions, these should be spaced 6–8 weeks apart.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree