3 Tumors and Tumorlike Lesions of the Skeleton and Soft Tissues Primary bone tumors are rare compared to metastases (secondary bone tumors) and hematologic conditions which include plasmocytomas and malignant lymphomas (Chapter 4). Tumorlike lesions are distinctly different from primary bone tumors but are customarily discussed with them. Tumorlike lesions can spontaneously regress or cease to grow and never metastasize. They are relatively common, they often defy any radiologic classification, and only a few of them require therapy. Bone tumors must be distinguished from primary soft-tissue tumors (pages 200–203). The classification of bone tumors according to the World Health Organization (WHO) is summarized in Table 3.1, and rare conditions are deliberately excluded. This classification is based on the dominant matrix produced by the primary tumor. Since this approach is not universally applicable, the site of tumor origin is also embodied in the classification. There are many tumorlike lesions that mimic primary and secondary bone tumors. These lesions resemble a tumor radiographically (localized osteolytic destruction or sclerosis), but fail to fulfill the pathologic criteria for a tumor (Table 3.2).
General Considerations in Diagnosing Skeletal Tumors
 The matrix is the intercellular substance produced by mesenchymal cells and includes osteoid, cartilage, myxoid ground substance, and (fibrous) collagenous fibers.
The matrix is the intercellular substance produced by mesenchymal cells and includes osteoid, cartilage, myxoid ground substance, and (fibrous) collagenous fibers.
 The clinically most important tumorlike lesion is osteomyelitis, which is dealt with separately under Infections in Chapter 2.
The clinically most important tumorlike lesion is osteomyelitis, which is dealt with separately under Infections in Chapter 2.
| Benign tumors | Malignant tumors |
|---|---|
| 1. Bone-producing tumors Osteoid osteoma Osteoblastoma | Osteosarcoma (subclassifications) |
| 2. Cartilage-producing tumors Chondroma Osteochondroma Chondroblastoma Chondromyxoid fibroma | Chondrosarcoma |
| 3. Giant cell tumor | |
| 4. Tumors arising from the bone marrow | |
| Ewing sarcoma Hematologic tumors (Lymphoma, plasmocytoma, see Chapter 4) | |
| 5. Vascular tumors Hemangioma Lymphangioma Glomus tumor | Hemangiopericytoma Hemangioendothelioma Angiosarcoma |
| 6. Connective tissue tumors Lipoma Desmoplastic fibroma Benign fibrous histiocytoma | Fibrosarcoma Malignant fibrous histiocytoma |
| 7. Other tumors | Chordoma Adamantinoma of the long tubular bones |
|
The Role of the Radiologist in Evaluating Skeletal Lesions Suspicious for Tumors
- After a lesion has been detected, distinguished from a normal variant, and categorized as pathological, a reasonable differential diagnosis of three most likely possibilities should be offered. In many cases, the lesion can only be classified as ‘probably benign’ or ‘probably malignant.’ If a lesion is judged by the examiner on the basis of his or her experience as unequivocally benign, this should be stated as such to avoid unnecessary biopsy or surgical therapy.
- The location of the lesion should be described to help plan the safest approach for biopsy or surgical excision. Depending on the presumptive nature of the lesion (benign versus malignant), either biopsy or primary excision should be considered in consultation with the surgeon. The radiologist should be conversant with the staging of primary bone tumors and with the imaging modalities most suitable for staging the individual case. A biopsy should be obtained only after a thorough imaging evaluation. It should be remembered that an open biopsy alters the MRI signal. Therefore, definitive diagnosis in patients who have had biopsies becomes more difficult.
- The radiologist should know the imaging modality suitable for monitoring any therapy and be familiar with the practical intervals of follow-up examinations.
- A correct diagnosis and rational therapeutic strategy for primary bone tumors can only be accomplished by the close cooperation of the radiologist, the surgeon, and the pathologist.
The Role of Diagnostic Imaging
All imaging methods play a role in diagnosing and staging primary and secondary bone tumors (metastases).
After radiographic or scintigraphic detection of an osseous lesion that could be a tumor, its radiographic features should be analyzed. These include:
- type of destruction,
- margin of the lesion,
- cortical changes,
- periosteal reaction,
- soft-tissue infiltration or mass.
This analysis generally determines the biologic activity (inactive, active, aggressive). This information coupled with the type of matrix production can often provide a definitive diagnosis.
The following lesions should be recognized and distinguished from other conditions:
- Inactive and benign ‘leave-me-alone’ lesions not requiring any therapy. Typical examples are bone infarcts, non ossifying fibromas, and bone islands.
- Bone lesions highly likely to be benign, which do not need immediate histologic evaluation and can be observed. Examples are fibrous dysplasia and post-traumatic myositis ossificans. Histologic confirmation is needed in individual cases only if an aggressive clinical course ensues after the lesion is discovered.
- Lesions that are probably benign and are unresectable for anatomic reasons regardless of the histologic finding. Examples are enchondromas and osteochondromas.
- All lesions of undetermined biologic activity or categorized as aggressive (perhaps malignant). These lesions must be biopsied after further diagnostic imaging and staging. Going straight to surgical resection is justified only if malignancy could be excluded by imaging.
 A suspected primary bone tumor or, less frequently, a tumorlike lesion of unknown nature should undergo an open surgical biopsy since a percutaneous biopsy may fail to obtain enough material for histologic evaluation. This does not apply to lesions suspected to be metastases, plasmocytoma, malignant lymphoma, or an inflammatory process. In these instances, percutaneous biopsy is more reasonable and less invasive.
A suspected primary bone tumor or, less frequently, a tumorlike lesion of unknown nature should undergo an open surgical biopsy since a percutaneous biopsy may fail to obtain enough material for histologic evaluation. This does not apply to lesions suspected to be metastases, plasmocytoma, malignant lymphoma, or an inflammatory process. In these instances, percutaneous biopsy is more reasonable and less invasive.
Ten Rules for Classifying Skeletal Lesions Suspicious for Tumors (according to Manaster)
Analysis of unknown skeletal lesions using the following ten determinants should lead to a definitive diagnosis, suggest a presumptive diagnosis or, at least, offer a limited and reasonable differential diagnosis.
- Age of the patient: Many tumors have a typical age distribution. This applies to Ewing sarcoma (childhood and adolescence), aneurysmal bone cyst (adolescence and young adulthood), osteosarcoma (childhood to young adulthood), and several sarcomas, such as chondrosarcoma and malignant fibrous histiocytoma (after the age of 30 years). Metastases and plasmocytomas usually do not occur before the fourth decade, with the notable exception of breast cancer metastases, which can occur at a younger age.
- Soft-tissue involvement: Cortical destruction with tumor extension into the soft tissue generally indicates a more aggressive growth and suggests a malignant lesion.
Caution: Aneurysmal bone cyst and eosinophilic granuloma are notable exceptions. Lesions with pathologic fractures may also present diagnostic problems.
A primary soft-tissue tumor is best demonstrated by MRI. Except for very aggressively infiltrating tumors, soft-tissue tumors respect the fascias for quite some time (in contrast to soft-tissue infection).
- Growth pattern: Analyzing the bone lesion (pattern of bone destruction, cortical involvement, periosteal reaction, etc.) usually allows categorization into three groups of biologic activity:
a) slow growth (‘inactive’),
b) intermediate growth (‘active’),
c) fast growth (‘aggressive’).
- Size of the lesion: The size of the lesion coupled with the pattern of bone destruction frequently reveals additional information about the biologic nature of the lesion. Large lesions with cortical destruction and little periosteal reaction should, for all intents and purposes, be assumed to be malignant.
- Location of the lesion: Some tumors commonly involve particular bones; for example the adamantinoma almost exclusively occurs in the tibia. Other lesions occur predominately in a preferred location in a tubular bone; for example chondroblastoma, is always an epiphyseal tumor. Some tumors occur more frequently in the axial than in the appendicular skeleton. Furthermore, the location within the bone (central versus eccentric, epiphyseal versus diaphyseal) can suggest the diagnosis. This will be discussed in more detail in the appropriate sections.
- Zone of transition: The transition from abnormal, pathologic bone to normal bone provides an additional indication of the nature of the lesion. A wide and indistinct zone of transition invariably indicates an aggressive and rapidly growing lesion, while a narrow zone of transition, in particular one with a sclerotic rim, can be assumed to be slow growing.
- Margin of the lesion: Though the margin is included in the preceding rule (6, Zone of transition), it can be seen as a different entity. Despite a very thin and well-demarcated zone of transition, a lesion can still be malignant or aggressive. This is seen in plasmocytomas and giant cell tumors.
- Tumor matrix: A categorization of bone and cartilage-forming tumors is possible by analyzing the visible tumor matrix. Furthermore, MRI can distinguish between myxoid substances and water-containing components (cysts).
- Host response: The response of the host bone to the tumor is reflected by cortical reaction (cortical thickening, formation of a neocortex, destruction, infiltration) and by periosteal reaction.
- Monostotic, oligostotic, or polyostotic lesions: Benign polyostotic lesions may include fibrous dysplasia, Paget disease, eosinophilic granuloma, and enchondromas. Malignant polyostotic lesions include metastases, plasmocytoma, and lymphoma.
 By incorporating these analytical characteristics into the clinical findings, the correct histologic diagnosis can often be established. At least the growth pattern of the lesion (least aggressive, more aggressive, highly aggressive) should be determined. It should also be remembered that aggressive growth does not invariably imply malignancy; this is characterized by aggressively growing benign lesions such as infection, eosinophilic granuloma, and aneurysmal bone cyst.
By incorporating these analytical characteristics into the clinical findings, the correct histologic diagnosis can often be established. At least the growth pattern of the lesion (least aggressive, more aggressive, highly aggressive) should be determined. It should also be remembered that aggressive growth does not invariably imply malignancy; this is characterized by aggressively growing benign lesions such as infection, eosinophilic granuloma, and aneurysmal bone cyst.
Staging (Determining the Extent of the Lesion)
The Enneking method is the generally accepted staging systems for primary bone tumors, especially malignant tumors.
This method is based on the histologic grading of the tumor (benign, low-grade malignant, high-grade malignant), its anatomic extent (mostly as determined by imaging), and the presence of metastases (in the skeleton or, above all, in the lung). These three factors are combined to plan the treatment strategy for a particular bone or soft-tissue tumor. The major role of the imager is to determine the local extent of the tumor and to search for skeletal metastases by scintigraphy and pulmonary metastases by chest CT.
A distinction should also be made between the intra- and extracompartmental locations of the tumor.
Intracompartmental lesions are defined as follows:
- All lesions confined to the subcutaneous fat (soft-tissue tumors).
- All lesions confined to the parosteal soft tissues. These tumors displace the muscles without infiltrating either muscle or osseous cortex.
- All bone tumors that are intracortical, i.e., the cortex or a surrounding neocortex is still visible.
- All soft-tissue tumors confined to a muscle compartment. The fascial planes enclosing these compartments are not violated. Examples of recognized muscle compartments are the posterior compartment of the calf, the anterior compartment of the calf, and the dorsal compartment of the forearm.
Extracompartmental lesions are defined as follows:
All tumors that violate any of the above-mentioned compartments or that abut or invade major neurovascular bundles. In addition, tumors are considered primary extracompartmental if located at a site without natural barriers.
These anatomic sites include mid-hand, midfoot, hind foot, popliteal fossa, femoral triangle, and axilla. Any tumor (bone or soft-tissue tumor) with articular extension is also considered extracompartmental.
Relative Values of the Different Imaging Modalities for Tissue Diagnosis, Assessing Biologic Activity, and Staging
Conventional Radiography
Radiography remains in the forefront of detecting skeletal lesions. Furthermore, it is the primary imaging modality to suggest the tissue diagnosis and to judge the biologic nature of tumorous lesions. For tumorlike or benign lesions, it can determine their extent.
Scintigraphy
Tc 99 m diphosphonate scintigraphy of the skeleton is used primarily to establish multifocality such as that seen with osseous metastases, and to monitor osseous lesions following therapy. Only a few entities, such as Paget disease and osteoid osteoma, exhibit a diagnostic pattern of bone uptake. The intensity of uptake within a lesion is not a reliable differentiating imaging finding.
CT
Although the role of CT for staging malignant tumors has decreased with the growth of MRI, it remains the standard method of evaluating uncertain radiographic and scintigraphic findings in the spine and pelvis. Some comparison studies could demonstrate that CT has the same accuracy as MRI for staging lesions as intra- versus extracompartmental in location. Because of its sensitivity in detecting calcifications and ossifications, CT is superior to conventional radiography in determining the tumor matrix and can add to the differential diagnostic assessment.
Sonography
Sonography is an acceptable bedside method for the detection and further evaluation of an undetermined swelling of the peripheral soft tissues, and supplements palpation and inspection. Ultrasonography is also suitable to guide percutaneous biopsies, especially of lesions in the peripheral soft tissues. Color Doppler duplex sonography can also provide differential diagnostic criteria, distinguishing, for instance, a solid tumor from a hematoma.
Angiography
Angiography has become obsolete for detecting and staging tumors and for offering differential diagnostic information. Even establishing the relationship between the neurovascular compartments and tumors has been taken over by MRI. Angiography still plays a role if embolization is contemplated (e.g., preoperative embolization of metastases or embolization of soft-tissue hemangiomas).
MRI
MRI is considered the gold standard for staging malignant and benign tumors. This applies to the entire skeleton, but above all to the extremities. Furthermore, MRI can provide a variety of differential diagnostic criteria by means of the different signal intensities and contrast enhancement. This application has increased significantly in recent years, showing a definite change from the initial verdict that MRI, like scintigraphy, provided only ‘non-specific’ data.
Technical Considerations: Differential diagnostic criteria are furnished by the signal intensities of the T1-weighted and T2-weighted SE sequences. The STIR sequence is a very good screening sequence. After intravenous administration of contrast medium, the Tl-weighted sequences, preferably with fat suppression, generally add useful clues. Depending on the size of the tumor, large body coils or special surface coils, adapted to the anatomy, are applied. MRI has the advantage of multiplanar imaging, providing a comprehensive assessment of the transitional zone between tumor and healthy tissue.
Caution: If a bone tumor is suspected, the MRI findings must be correlated with those from conventional radiography.
 MRI is frequently unable to distinguish between benign and malignant bone or soft-tissue tumors. It is, however, quite reliable in distinguishing tumors from inflammatory and ischemic lesions. This is especially true in differentiating between tumor, osteomyelitis, and bone infarct, which is of profound clinical importance.
MRI is frequently unable to distinguish between benign and malignant bone or soft-tissue tumors. It is, however, quite reliable in distinguishing tumors from inflammatory and ischemic lesions. This is especially true in differentiating between tumor, osteomyelitis, and bone infarct, which is of profound clinical importance.
|
|
|
The detection of peritumoral edema, within or outside the bone, is a non-specific finding, but can aid in detecting tumors and arriving at a tissue diagnosis:
- Peritumoral edema is more frequent with malignant tumors than with benign ones, but it is not a reliably distinguishing finding.
- Edema can occur with infections and trauma and, rarely, with acute bone infarcts. A space-occupying lesion (tumor) can generally be identified against the surrounding edema. In many cases, this finding can help in the tentative differentiation between tumor, inflammation, and infarct.
- It should be kept in mind that clusters of tumor cells (only microscopically detectable) can be embedded in the edema surrounding the tumor. This is important when determining the margins of resection, which should generally include the edematous zone.
- The most sensitive detection for a peritumoral edema is rendered by the STIR sequence and the T1-weighted SE sequence with fat suppression, together with the administration of contrast medium. Remember that edema does not enhance.
Staging and MRI
The most elemental question in staging is whether the lesion is intracompartmental or extracompartmental, and this is determined by MRI in the majority of cases. The multiplanar capability of MRI also makes it the modality of choice for determining the osseous and soft-tissue extent of the tumor and the relationship of the tumor to fascia and adjacent articular structures. Furthermore, MRI can reliably delineate the relationship of the tumor to the major neurovascular compartments.
The intraosseous extent of a lesion is best determined on the T1-weighted SE images. The imaging plane should be determined by the anatomy (e.g., coronal or sagittal planes for tubular bones). The T2-weighted images, especially the so-called turbo SE sequences, are not suitable for determining the osseous extent of the lesion. The administration of contrast medium is only meaningful if the T1-weighted sequences can be combined with fat suppression.
The soft-tissue extent is best assessed on STIR sequences because these sequences achieve a high contrast between tumor, muscle, and fat. The T1-weighted SE sequences, excellent for anatomic detail, are most helpful when obtained with fat suppression and after administration of contrast medium.
When assessing the relationship between the tumor and the adjacent joints as part of the staging, the following questions must be addressed:
- Is the joint involved?
- Do the tumor margins abut the joint, producing a sympathetic effusion? Remember, however, that the effusion may not be induced by the tumor itself. A tumor-induced effusion occurs only after direct tumorous invasion of the capsule.
- Is the tumor intracapsular?
- Is the tumor growth transarticular?
Tumors can extend directly to the subchondral interface, as is the situation with aneurysmal bone cysts, giant cell tumors, or chondroblastoma. This is relevant for planning the surgical approach since the articular surface might not be preserved in many of these cases, and it might be necessary to modify the surgical technique, possibly resorting to an endoprosthesis.
Therapeutic Strategies of Bone and Soft-tissue Tumors
The different therapeutic strategies, primarily surgical, are determined by the tissue diagnosis and the extent of the lesion. Intralesional excision (curettage) is to be distinguished from marginal excision. An additional surgical strategy is the wide excision (4 cm beyond the tumor margin) or radical resection (resection of the tumor, including the entire surrounding musculature and surrounding bone). Malignant bone and soft-tissue tumors generally undergo preoperative and often also postoperative chemotherapy. This is especially true in tumors such as Ewing sarcoma and osteosarcoma. Radiotherapy of primary bone and soft-tissue tumors is less commonly performed today. However, palliative radiation remains effective for certain bone and soft-tissue metastases.
Primary Bone Tumors
Bone-Producing Tumors
Osteoid Osteoma
 The tumor consists of highly vascularized stroma with osteoid and immature bone.
The tumor consists of highly vascularized stroma with osteoid and immature bone.
 Severe pain (especially at night), relieved by aspirin (less than 50% of cases).
Severe pain (especially at night), relieved by aspirin (less than 50% of cases).
Age: First through third decade.
Location: The femur (30%) and tibia (25%) are most frequently involved. The lesion is eccentric, frequently intra-cortical (80–90%).
Therapy: Surgical excision (immediate pain relief indicates successful removal). Percutaneous excision or alcohol ablation can be used in selected cases.
 Clinically relevant locations are intracapsular osteoid osteomas (joint pain) and the spine (reactive scoliosis).
Clinically relevant locations are intracapsular osteoid osteomas (joint pain) and the spine (reactive scoliosis).
 The tumor causes focal bone destruction (nidus) that is surrounded by varying degrees of sclerosis (Figs. 3.1–3.3). The osteoid produced by the tumor can ossify, leading to diffuse or punctate areas of increased density within the osteolytic destruction. A severely ossified nidus can merge with the surrounding sclerosis. The following are possible locations for the nidus:
The tumor causes focal bone destruction (nidus) that is surrounded by varying degrees of sclerosis (Figs. 3.1–3.3). The osteoid produced by the tumor can ossify, leading to diffuse or punctate areas of increased density within the osteolytic destruction. A severely ossified nidus can merge with the surrounding sclerosis. The following are possible locations for the nidus:
- Intracortical, surrounded by severe reactive ovoid or biconvex new bone formation and occurring in the cortex with possible bone marrow extension (Fig. 3.2).
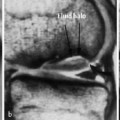
- Medullary, (e.g., in the hands or feet), generally with less severe surrounding sclerosis and frequently demarcated as a sclerotic structure (Fig. 3.3).
- Intraspinal, (often in the vertebral arch) where the nidus and sclerosis are barely separable.
- Intra-articular, (e.g., hip joint), often seen as a relatively subtle, subarticular, round to ovoid radiolucency. The lesion can also bulge into the joint and be surrounded solely by a subtle sclerotic rim.
 Localized, frequently eccentric uptake of severe intensity, coupled with radiography, usually allows a definitive diagnosis to be rendered.
Localized, frequently eccentric uptake of severe intensity, coupled with radiography, usually allows a definitive diagnosis to be rendered.
 Same as the radiographic findings, with the advantage that the lesion is delineated without superimposed osseous structures.
Same as the radiographic findings, with the advantage that the lesion is delineated without superimposed osseous structures.
 The nidus is of low signal intensity on T1-weighted images, but of variable signal intensities on T2-weighted images (depending on the extent of the calcifications) (Fig. 3.4).
The nidus is of low signal intensity on T1-weighted images, but of variable signal intensities on T2-weighted images (depending on the extent of the calcifications) (Fig. 3.4).
Caution: Extensive edema surrounding the lesion with soft-tissue involvement can cause confusion with a more aggressive lesion.
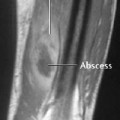
 Brodie’s abscess: small abscesses appear identical to those of osteoid osteoma.
Brodie’s abscess: small abscesses appear identical to those of osteoid osteoma.
Bone island: osteoid osteoma almost always induces an osseous reaction, whereas the bone island does not.
Osteoblastic metastases: especially in the spine, when randomly distributed multiple lesions are shown on the bone scan.
Chronic osteomyelitis and fractures: Diffuse, indistinctly demarcated osteoid osteomas of the foot and hand skeleton can mimic these lesions.
Osteoblastoma
 The pain is less severe than that experienced with osteoid osteoma.
The pain is less severe than that experienced with osteoid osteoma.
Location: About 40% of osteoblastomas are in the spine; the rest are found in the tubular bones, skull, and other bones.
Therapy: Curettage.
 Relevant findings:
Relevant findings:
- Osteolytic destruction, usually sharply demarcated. Intralesional calcifications are frequent (Fig. 3.5).
- Surrounding sclerosis is induced less frequently than with the osteoid osteoma.
- Cup-like cortical defect if extrinsically located.
- Osseous extension is frequent.
 Important for the assessment of the tumor margin and the method of choice for the spine (Fig. 3.6).
Important for the assessment of the tumor margin and the method of choice for the spine (Fig. 3.6).
 Osteosarcoma: Individual cases can be radiographically identical. Brown tumors of hyperparathyroidism: Differentiation by clinical findings and laboratory parameters. Aneurysmal bone cyst: Cystic cavities, trabeculated.
Osteosarcoma: Individual cases can be radiographically identical. Brown tumors of hyperparathyroidism: Differentiation by clinical findings and laboratory parameters. Aneurysmal bone cyst: Cystic cavities, trabeculated.
Caution: Osteoblastoma and aneurysmal bone cysts can co-exist.
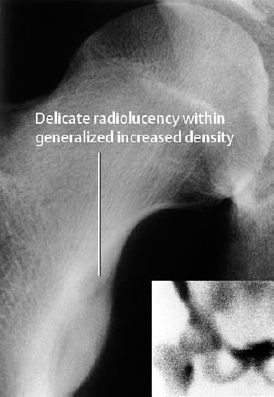
Fig. 3.1 Osteoid osteoma in the lesser trochanter of the femur.
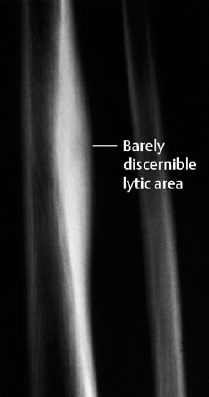
Fig. 3.2 Osteoid osteoma of the tibia with typical fusiform periosteal reaction.
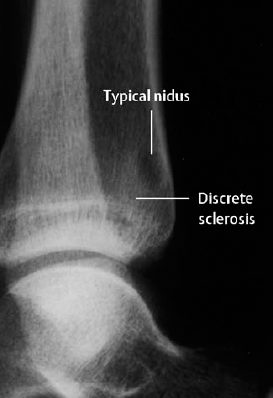
Fig. 3.3 Osteoid osteoma of the distal tibia surrounded by only a minimal reaction.
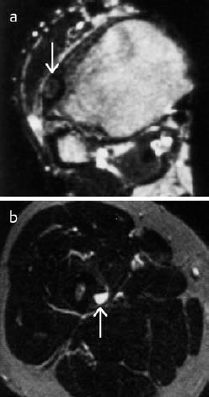
Fig. 3.4 MRI of osteoid osteoma, T2-weighted images.
a Lesion of relatively low signal intensity corresponding to delicate calcifications not yet visible radiologically.
b Lesion of high signal intensity in the cortex.
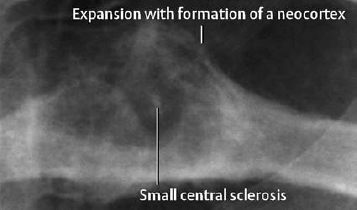
Fig. 3.5 Osteoblastoma of the rib. The margin is not completely discernible (→CT).
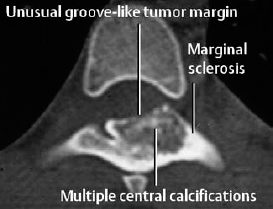
Fig. 3.6 Osteoblastoma of the vertebral arch.
Osteosarcoma
Synonym: Osteogenic sarcoma
 The tumor cells form osteoid. Histologically, subclassifications are made on the basis of the dominating tissue differentiation (osteoblastic, chondro-blastic, fibroblastic, etc.). Numerous variants exist:
The tumor cells form osteoid. Histologically, subclassifications are made on the basis of the dominating tissue differentiation (osteoblastic, chondro-blastic, fibroblastic, etc.). Numerous variants exist:
- ‘conventional’ osteosarcoma (about 75%, see below),
- periosteal osteosarcoma (see below),
- parosteal osteosarcoma (see below),
- telangiectatic osteosarcoma (highly malignant, purely lytic osteosarcoma),
- ‘low-grade’ osteosarcoma (prognostically favorable, slow-growing osteosarcoma),
- osteosarcoma of older patients (above 60 years of age, e.g., associated with Paget disease, post-radiotherapy),
- soft-tissue osteosarcoma (beginning after the age of 40 years).
 Slowly worsening local pain.
Slowly worsening local pain.
Age: Two incidence peaks: age 10–25 years and age 60–80 years, but can occur at any age.
Location: The tumor can arise in any bone but over 80% occur in the long tubular bones (femur, tibia) and involve the metaphysis in the majority of cases.
Prognosis and therapy: The five-year survival rate depends on the type of tumor and on the time of the diagnosis, ranging between 30 and 80%. Preoperative chemotherapy is followed by amputation or wide resection. The surgical approach depends on the local tumor stage, best shown by MRI, and on the presence of metastases shown by bone scintigraphy and chest CT.
‘Conventional’ Osteosarcoma
 The radiographic findings are variable.
The radiographic findings are variable.
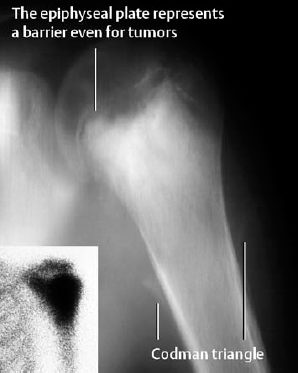
Mixed osteolytic/osteoblastic morphology: This is the most frequent manifestation. Radiographically, osteolytic and osteoblastic changes are found together, with the osteoblastic changes corresponding to mineralized osteoid. The degree of osteolytic and osteoblastic changes varies from case to case. The tumor margins are indistinct and periosteal new bone formation, characteristically spiculated, is invariably present. The Codman triangle, a manifestation of periosteal elevation, is often observed. Furthermore, lamellated, intermittently disrupted periosteal bone formation is consistently observed (Fig. 3.7b).
Osteolytic osteosarcoma (about 10% of cases): moth-eaten pattern of destruction, almost always with periosteal reaction (Fig. 3.7a).
Osteoblastic osteosarcoma (about 10% of cases): characterized by ivory or dense cloudlike sclerotic tumor mass (Fig. 3.8). The tumor can be confined to the outline of the affected bone and sharply demarcated from the surrounding spongiosa. Any extension of the tumor into the soft tissues induces a periosteal reaction.
 This is important to exclude metastases and to monitor the tumor’s response to therapy.
This is important to exclude metastases and to monitor the tumor’s response to therapy.
 This is superior to conventional radiography in differentiating reactive changes (e.g., periosteal response) from the tumor itself
This is superior to conventional radiography in differentiating reactive changes (e.g., periosteal response) from the tumor itself
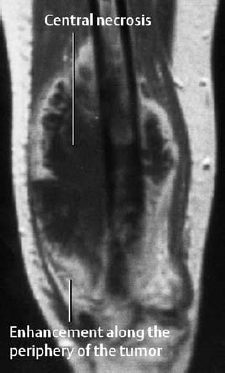
 The signal pattern is determined by the degree of ossification of the matrix. MRI is the method of choice for staging lesions in tubular bones (intramedullary extension, soft-tissue infiltration, intracompartmental versus extracompartmental tumor component, joint involvement) (Fig. 3.9).
The signal pattern is determined by the degree of ossification of the matrix. MRI is the method of choice for staging lesions in tubular bones (intramedullary extension, soft-tissue infiltration, intracompartmental versus extracompartmental tumor component, joint involvement) (Fig. 3.9).
 Ewing sarcoma: No differentiation if there is no matrix produced by the osteosarcoma or if it is only faintly ossified.
Ewing sarcoma: No differentiation if there is no matrix produced by the osteosarcoma or if it is only faintly ossified.
Cortical desmoid: The eccentric location at the distal femoral metaphysis is the key to the diagnosis of a cortical desmoid.
Chronic osteomyelitis: Usually differentiated by MRI by showing a solid tumor that is absent in osteomyelitis.
Chondrosarcoma: The considerable amount of cartilage produced by some osteosarcomas might preclude the differentiation between a so-called ‘chondroblastic osteosarcoma’ and a chondrosarcoma.
Glossary
Parosteal osteosarcoma (juxtacortical osteosarcoma, parosseous osteosarcoma):
This well-differentiated osteosarcoma has its epicenter adjacent to the periosteum and has a much better prognosis than the ‘conventional’ osteosarcoma.
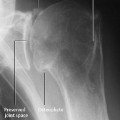
Parosteal osteosarcoma appears as a lobulated density along the surface of the metaphyseal cortex of the tubular bone and leads to thickening and deformity of the cortex. Only 10% of the tumors extend into the bone marrow space (Fig. 3.10).
The differential diagnosis includes myositis ossificans, juxtacortical osteoma, and other osseous sarcomas.
Periosteal Osteosarcoma: Like the parosteal osteosarcoma, this is a surface lesion, preferentially involving the tibial and femoral diaphyses. It is dominated by a soft-tissue mass with a tumor matrix that is less mineralized than the parosteal osteosarcoma. MRI is essential for tumor staging.
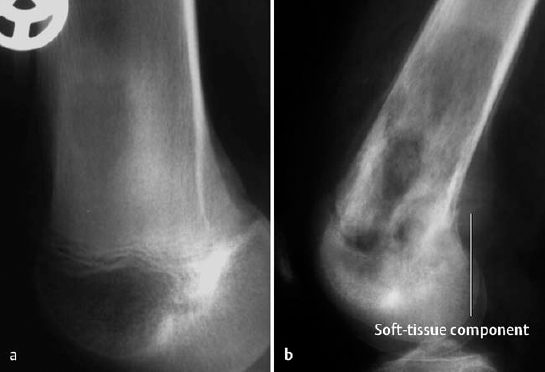
For left:
Fig. 3.8 Predominately osteoblastic osteosarcoma.
Near left:
Fig. 3.9 MRI of an osteosarcoma.
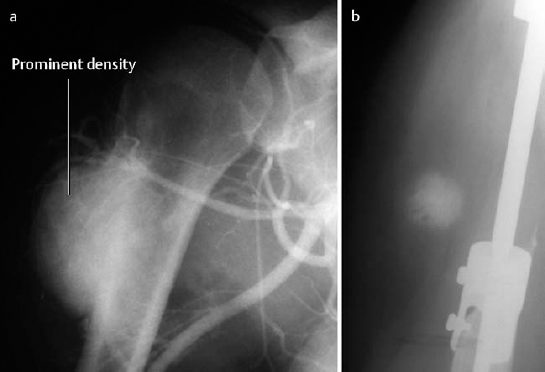
Fig. 3.10
a Parosteal osteosarcoma (frame from an angiographic series).
b Local soft-tissue metastasis after tumor excision and insertion of a prosthesis.
Cartilage-Producing Tumors
Osteochondroma
Synonym: Cartilaginous exostosis
 The cartilaginous center ossifies with the outer layer, remaining as a non-ossified layer of cartilage of varying thickness forming a cartilaginous cap. Because of its self-limiting growth, it is more appropriately characterized as a tumorlike lesion.
The cartilaginous center ossifies with the outer layer, remaining as a non-ossified layer of cartilage of varying thickness forming a cartilaginous cap. Because of its self-limiting growth, it is more appropriately characterized as a tumorlike lesion.
 No pain, usually an incidental finding, unless there is mechanical pressure on a neurovascular bundle or formation of a ‘pseudobursa’.
No pain, usually an incidental finding, unless there is mechanical pressure on a neurovascular bundle or formation of a ‘pseudobursa’.
Age: First to third decade of life.
Location: Metaphyseal region of the tubular bones.
Therapy: None, or simple excision, depending on clinical symptoms and location.
 The osteochondroma can be pedunculated, arising by a stalk from the cortex (cauliflower-type, Fig. 3.11), or sessile, with its broad base incorporated in the bone and associated with loss of a discernible cortex (see ‘localized bone thickening’, Fig. 3.12). Large osteochondromas can deform the bone (Fig. 3.13). Calcifications (linear, geographic, coarse, irregular) are always detectable, unless the osseous chondroma has matured and is completely incorporated into the osseous structures (Fig. 3.14).
The osteochondroma can be pedunculated, arising by a stalk from the cortex (cauliflower-type, Fig. 3.11), or sessile, with its broad base incorporated in the bone and associated with loss of a discernible cortex (see ‘localized bone thickening’, Fig. 3.12). Large osteochondromas can deform the bone (Fig. 3.13). Calcifications (linear, geographic, coarse, irregular) are always detectable, unless the osseous chondroma has matured and is completely incorporated into the osseous structures (Fig. 3.14).
- It is clearly demarcated from the surrounding soft tissues, but frequently irregularly outlined.
- No sclerotic margins.
 To delineate the radiographic findings without superimposed structures and, especially in the central skeleton, to determine the site of origin to better advantage (superior to MRI). The delineation of the cartilaginous cap is poor.
To delineate the radiographic findings without superimposed structures and, especially in the central skeleton, to determine the site of origin to better advantage (superior to MRI). The delineation of the cartilaginous cap is poor.
 The cartilaginous cap of superficial osteochondromas can be superbly visualized.
The cartilaginous cap of superficial osteochondromas can be superbly visualized.
 Like CT, it confirms the diagnosis by showing the continuity of the bone marrow with the marrow-filled internal space of the mature osteochondroma. MRI is the optimal method for determining the thickness of the cartilaginous cap (Fig. 3.16).
Like CT, it confirms the diagnosis by showing the continuity of the bone marrow with the marrow-filled internal space of the mature osteochondroma. MRI is the optimal method for determining the thickness of the cartilaginous cap (Fig. 3.16).
 A cartilaginous cap measuring more than 2 cm suggests sarcomatous degeneration. Sarcomatous degeneration can be expected to occur in approximately 1% of solitary and 10–15% of multiple osteochondromas.
A cartilaginous cap measuring more than 2 cm suggests sarcomatous degeneration. Sarcomatous degeneration can be expected to occur in approximately 1% of solitary and 10–15% of multiple osteochondromas.
The multiple osteochondromas associated with multiple hereditary exostosis, an autosomal dominant disorder, are more often sessile than pedunculated.
 Pedunculated cauliflower exostoses:
Pedunculated cauliflower exostoses:
- parosteal osteosarcoma: broader base, thick matrix,
- juxtacortical (parosteal) osteoma: homogeneous osseous matrix (Fig. 3.15),
- chondrosarcoma: thicker cartilaginous cap!
- myositis ossificans: ossification in the soft tissues that is separated from the cortex. This is best shown by CT or MRI.
Broad-based exostoses:
- fibrous dysplasia: no fatty marrow within the lesion.
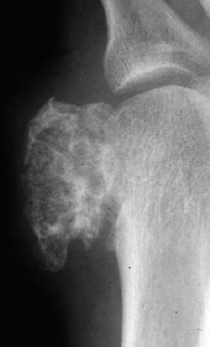
Fig. 3.11 Osteochondroma. Cauliflower exostosis arising from the first metatarsal bone.
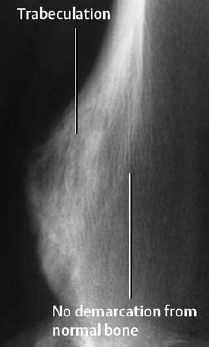
Fig. 3.12 Osteochondroma. Broad-based excrescence arising from the underlying bone. The cartilage matrix has been transformed into trabecular bone through ossification.
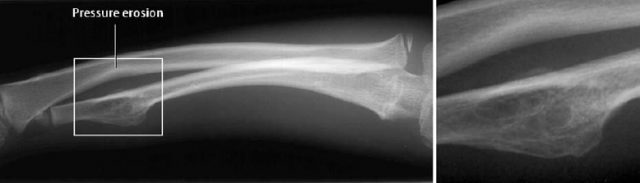
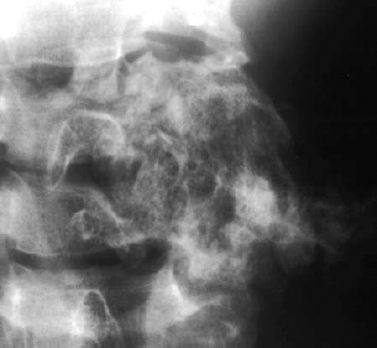
Fig. 3.14 Osteochondroma of the cervical spine. The point of origin of the tumor can no longer be determined. Coarsened trabecular pattern, stippled calcifications, and slow expansile growth permit the diagnosis.
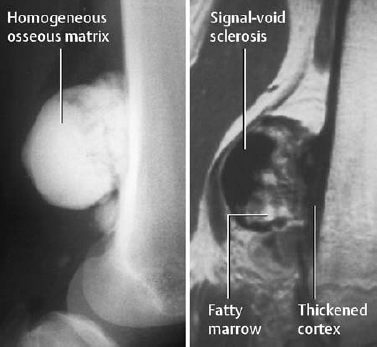
Fig. 3.15 Parosteal osteoma. It can be differentiated from an osteochondroma by the underlying cortex. The fatty marrow within the internal tumor space is noteworthy (MRI:T1-weighted image).
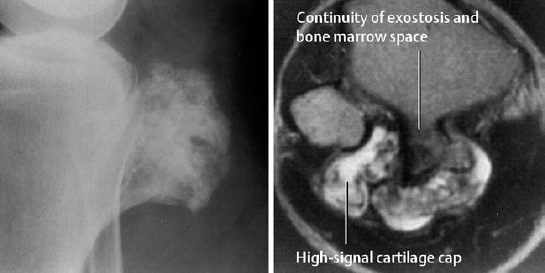
Fig. 3.16 Osteochondroma with coarsened trabeculation (compare with Fig. 3.15). The exostosis is well demarcated from the soft tissues. Only MRI (T2-weighted image) shows the entire 1cm thick bright cartilage cap. Notice that the cortex and osteochondroma are directly connected.
Enchondroma
Synonym: Chondroma
 The tumor consists of mature cartilage. A distinction can be made between the central enchondroma and the eccentrically growing enchondroma with cortical destruction and formation of a ‘subperiosteal enchondroma’.
The tumor consists of mature cartilage. A distinction can be made between the central enchondroma and the eccentrically growing enchondroma with cortical destruction and formation of a ‘subperiosteal enchondroma’.
 No pain (a feature distinguishing it from chondrosarcoma).
No pain (a feature distinguishing it from chondrosarcoma).
Age: Usually discovered before age 40 years.
Location: All bones with enchondral ossification can be affected. The tumor has a predilection for the tubular bones of the hands and feet. In the long bones, the enchondroma is characteristically located in the metadiaphyseal region.
Therapy: Lesions of the tubular bones should be left alone. Curettage should pe performed only if the lesion is painful or if it is located in the axial skeleton.
 Round or ovoid radiolucencies, often with a scalloped border, or a lesion with a matrix of mixed coarse, ringlike, or confluent calcifications. A sclerotic margin is not always detectable. The lesion may thin and scallop the cortex because of endosteal erosion. Periosteal reaction and bone expansion are possible (Figs. 3.17, 3.18).
Round or ovoid radiolucencies, often with a scalloped border, or a lesion with a matrix of mixed coarse, ringlike, or confluent calcifications. A sclerotic margin is not always detectable. The lesion may thin and scallop the cortex because of endosteal erosion. Periosteal reaction and bone expansion are possible (Figs. 3.17, 3.18).
 Superior visualization of the calcifications shows the same diagnostic information as that of conventional radiography and demonstrates calcification to better advantage.
Superior visualization of the calcifications shows the same diagnostic information as that of conventional radiography and demonstrates calcification to better advantage.
 Hypointense signal on T1-weighted images. The signal intensity on T2-weighted images depends on the degree of calcification. Non-calcified enchondromas can be so bright that the differentiation from a cyst can be difficult. IV administration of contrast medium can be helpful by disclosing the typical image of a lobulated lesion with septation (Fig. 3.19).
Hypointense signal on T1-weighted images. The signal intensity on T2-weighted images depends on the degree of calcification. Non-calcified enchondromas can be so bright that the differentiation from a cyst can be difficult. IV administration of contrast medium can be helpful by disclosing the typical image of a lobulated lesion with septation (Fig. 3.19).
 Hands and feet:
Hands and feet:
- Giant cell tumor of the tendon sheath (soft-tissue tumor with extrinsic bone erosion).
- Aneurysmal bone cyst, juvenile bone cyst (MRI can be helpful in the differentiation).
Other locations:
- Bone infarct: The calcifications are more peripheral than central (‘encasing the lesion’). MRI may be helpful in some cases.
- Chondrosarcoma: A diagnostic dilemma! (See chondrosarcoma). Differentiation from a low-grade chondrosarcoma is often very difficult.
 Remember: Multiple enchondromatosis = Oilier disease. Multiple enchondromatosis with soft-tissue hemangiomas = Maffucci syndrome.
Remember: Multiple enchondromatosis = Oilier disease. Multiple enchondromatosis with soft-tissue hemangiomas = Maffucci syndrome.
Chondroblastoma
Synonym: Codman tumor
 Benign tumor consisting of chondroblasts and numerous giant cells.
Benign tumor consisting of chondroblasts and numerous giant cells.
 Mild, increasing pain. Possible joint effusion.
Mild, increasing pain. Possible joint effusion.
Age: About 75% of cases occur between 10 and 25 years of age.
Location: All bones with enchondral calcification can be affected (especially the humerus). Characteristically, the lesion is centrally located within the epiphysis and may have metaphyseal extension.
Therapy: Curettage. There is a recurrence rate of 15%.

- Round, solitary radiolucency 1–4 cm in diameter with sharp demarcation and sclerotic rim (Fig. 3.20).
- Intralesional calcifications in about 50% of cases.
- Osseous expansion in flat bones. This occurs less frequently in tubular bones.
- Periosteal reaction in about 40% of cases.
 Delineation of the radiographic changes where there is superimposition of other structures (Fig. 3.21).
Delineation of the radiographic changes where there is superimposition of other structures (Fig. 3.21).
 Findings similar to those of enchondroma, but the lesion may be surrounded by edema that can penetrate the cortex.
Findings similar to those of enchondroma, but the lesion may be surrounded by edema that can penetrate the cortex.

- Giant cell tumor (no calcification).
- Chondrosarcoma (soft-tissue extension).
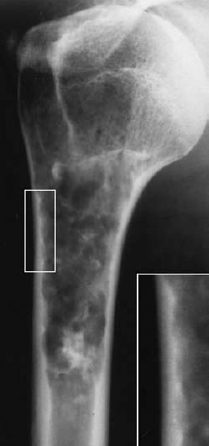
Fig. 3.17 Classic enchondroma with scalloping of the cortex (see magnified view).
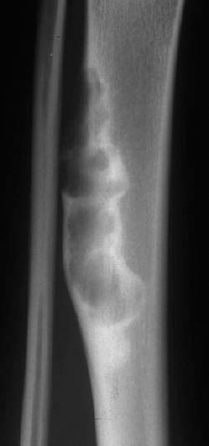
Fig. 3.18 Periosteal chondroma. This eccentric lobulated tumor has a thick sclerotic rim which is somewhat atypical.
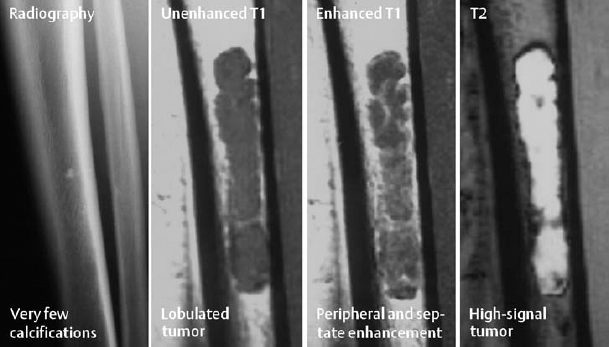
Fig. 3.19 Enchondroma. The differentiation from a cyst on MRI can be difficult. Conventional radiography provides the definitive findings of calcifications and absent marginal sclerosis.
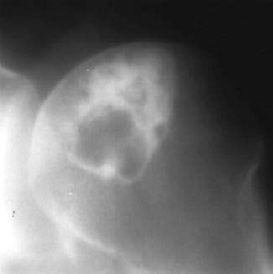
Fig. 3.20 Chondroblastoma. Classic location and thick sclerotic rim.
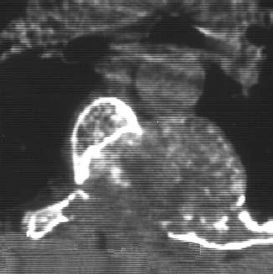
Fig. 3.21 Chondroblastoma of the posterior vertebral arch with intralesional calcification (axial CT).
Chondromyxoid Fibroma
This is a very rare benign cartilaginous tumor, characterized by an abundance of myxoid or chondroid intracellular substance. It occurs almost exclusively in the proximal tibia and usually presents radiographically as a sharply demarcated solitary radiolucency that rarely calcifies.
Chondrosarcoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 This is a benign, painful osseous lesion that is characterized by small size (less than 2 cm) and sharp demarcation, frequently surrounded by a sclerotic reaction.
This is a benign, painful osseous lesion that is characterized by small size (less than 2 cm) and sharp demarcation, frequently surrounded by a sclerotic reaction. 
 This is a benign bone-forming tumor larger than 2 cm (the so-called ‘big brother’ of the osteoid osteoma). It can be difficult to distinguish the aggressive type of this osteoblastoma from an osteosarcoma (rare).
This is a benign bone-forming tumor larger than 2 cm (the so-called ‘big brother’ of the osteoid osteoma). It can be difficult to distinguish the aggressive type of this osteoblastoma from an osteosarcoma (rare). 
 Osteosarcoma is the most common malignant primary bone tumor, most frequently occurring in children and adolescents.
Osteosarcoma is the most common malignant primary bone tumor, most frequently occurring in children and adolescents. 
 This is a very common benign osseous neoplasm that extends from the underlying bone.
This is a very common benign osseous neoplasm that extends from the underlying bone. 
 This is a benign cartilage-forming tumor, which may, in certain instances, be difficult to differentiate histologically from a low-grade chondrosarcoma.
This is a benign cartilage-forming tumor, which may, in certain instances, be difficult to differentiate histologically from a low-grade chondrosarcoma. 
 Chondroblastoma is found almost exclusively in the epiphyses of the tubular bones of adolescents before closure of the growth plate.
Chondroblastoma is found almost exclusively in the epiphyses of the tubular bones of adolescents before closure of the growth plate.