Primary tumors of the liver can be classified pathologically based on their cell of origin into epithelial tumors, arising from hepatocytes or biliary epithelium, and nonepithelial tumors, including mesenchymal tumors and lymphoma. Characteristic findings on MR imaging can be seen in many cases. This article reviews the MR imaging appearance of these tumors with pathologic correlation.
Primary tumors of the liver can be broadly classified pathologically based on their cell of origin. Epithelial tumors arise from hepatocytes or biliary epithelium and include the benign neoplasms or tumor-like lesions focal nodular hyperplasia (FNH), hepatocellular adenoma (HCA), and biliary cystadenoma; in addition to the malignant neoplasms hepatocellular carcinoma (HCC), fibrolamellar carcinoma, and intrahepatic cholangiocarcinoma (ICC). Nonepithelial tumors consist of lymphoma and mesenchymal tumors, including cavernous hemangioma, angiomyolipoma, solitary fibrous tumor (SFT), angiosarcoma, and hepatic epithelioid hemangioendothelioma. Characteristic findings on MR imaging can be seen in many cases. In this article we review the MR imaging appearance of these tumors with pathologic correlation.
Benign tumors
Epithelial
Focal nodular hyperplasia
Clinical and pathologic features
FNH is the second most common benign hepatic tumor following hemangioma, accounting for 8% of primary hepatic tumors. It is classified as a regenerative lesion rather than a neoplasm. It is thought to represent a hyperplastic response to a congenital or acquired arterial malformation. FNH with histologic characteristics of both FNH and HCA and atypical pathologic and imaging characteristics for FNH, such as heterogeneity and lack of a central scar, were previously categorized as telangiectatic FNH. Because of recent molecular evidence, they are now recognized as a subset of HCA.
Approximately 89% to 94% of FNH occur in women with a mean age at diagnosis of 38 years. The majority of patients are asymptomatic. In the remainder, abdominal pain and palpable mass are the most common symptoms. Reports of hemorrhage or rupture of FNH are extremely rare.
FNH are named for their nodular architecture, subdivided by fibrous septa that coalesce into a central or eccentric stellate scar. They are nonencapsulated, but sharply marginated with a lobulated contour. Prominent vessels cover their surface. Hemorrhage and necrosis are rare because their growth is usually proportional to their vascular supply. Their size ranges from 1 mm to 19 cm in diameter, with a mean of 5 cm. Approximately 20% are multiple and there are reported associations with other vascular malformations and neoplasms, including cavernous hemangiomas, which are present in 20% of patients with FNH.
Microscopically, FNH consist of hyperplastic hepatocytes that are arranged in two-cell thick hepatic plates separated by sinusoids containing endothelial cells and Kupffer cells. The fibrous septa contain numerous vessels, particularly thick-walled arteries with fibromuscular hyperplasia and intimal fibrosis, in addition to an inflammatory infiltrate. Bile ductules are present at the junction of the fibrous septa and hepatocytes; however, they do not connect to the intrahepatic bile ducts.
MR imaging features
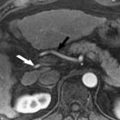
The diagnosis of FNH can be made with confidence when all typical MR imaging features are present on unenhanced and intravenous contrast-enhanced sequences ( Fig. 1 ). These include isointensity or hypointensity on T1-weighted sequences (90% to 100% of cases) and slight hyperintensity or isointensity on T2-weighted sequences (99% to 100% of cases) with homogeneous signal intensity except for a central scar. The scar is hyperintense on T2-weighted sequences because of the presence of vascular channels, bile ductules, and myxomatous tissue. After the administration of intravenous gadolinium, FNH typically demonstrate moderate-to-strong homogeneous arterial enhancement, with slight hyperintensity or isointensity to background liver in the portal venous and equilibrium phases (95% to 99% of cases). The central scar demonstrates initial relative hypointensity during the arterial phase of contrast enhancement, and subsequent delayed enhancement from the progressive accumulation of contrast within the fibrous tissue. Prominent peripheral draining veins or a dominant draining vein may be observed surrounding the lesion on equilibrium phase images (see Fig. 1 D).
Atypical imaging features are found in 21% to 57% of FNH. A central scar may not be visualized in up to 50% of cases, particularly in lesions less than 3 cm in diameter. Rarely, the scar may demonstrate low T2 signal intensity (1%) or lack of enhancement (1% to 2%) simulating fibrolamellar carcinoma. Other atypical features include hypoenhancement during any phase of contrast-enhanced imaging, heterogeneous signal intensity from hemorrhage, high T1 signal intensity from sinusoidal dilatation or steatosis, and a peripheral pseudocapsule of low T1 and high T2 signal intensity from adjacent compressed liver.
Liver-specific MR imaging contrast agents can be helpful in the diagnosis of FNH. Superparamagnetic iron oxide (SPIO) particles taken up by Kupffer cells lower the signal intensity of FNH on T2-weighted and T2-weighted sequences, although usually to a slightly less extent than normal liver, and improve visualization of the central scar. Hepatobiliary agents, such as mangafodipir or delayed imaging with gadobenate dimeglumine, result in isointensity or hyperintensity on T1-weighted images because FNH contains functioning hepatocytes and biliary ductules (see Fig. 1 E). In one study of gadobenate dimeglumine, 97% of FNH were hyperintense or isointense compared with 100% of HCA being hypointense on a 1 to 3 hour delayed T1 sequence. The central scar, hypointense during the hepatobiliary phase, was also better delineated.
Differential diagnosis
Focal hepatic lesions that may have a central scar or scar-like fibrosis include hemangioma, fibrolamellar carcinoma and HCC. Giant hemangiomas may contain focal fibrosis that simulates a central scar (discussed below). This is usually larger and more hyperintense on T2-weighted sequences compared with the scar of FNH and the contrast enhancement pattern of hemangiomas is distinctive. The scar of fibrolamellar carcinoma contains calcification in 55% and is typically hypointense on T2-weighted sequences. Both fibrolamellar carcinoma and HCC tend to be more heterogeneous than FNH because they commonly have intratumoral hemorrhage and necrosis.
Hypervascular liver lesions such as HCA and HCC may also be considered in the differential diagnosis of FNH, particularly when a central scar is not visualized. The distinction between these tumors is discussed in the following section on HCA.
Hepatocellular adenoma
Clinical and pathologic features
HCA is a rare benign neoplasm that most often arises in the setting of hormonal or metabolic stimulation. The most common cause is oral contraceptives, with an annual incidence of 3 to 4 per 100,000 in women on long-term contraceptives, compared with 1 per million in women who have not taken oral contraceptives or have taken them for less than 2 years. Risk increases with the duration of use and potency of oral contraceptives and appears to be lower with second and third generation lower dose formulations. Other risk factors for HCA include anabolic steroid use, pregnancy, gynecologic tumors, glycogen storage diseases (particularly type Ia, von Gierke disease), and galactosemia. Sporadic cases have been reported in men and women without any risk factors, but are rare.
Similar to FNH, HCA are usually found in women of childbearing age, with most in the third to fourth decades of life. The majority of patients are symptomatic, complaining of acute, episodic, or chronic abdominal pain, or a palpable mass. Only 5% to 10% of lesions are incidentally discovered. Twenty five percent present with tumor rupture and subcapsular or intraperitoneal hemorrhage, which occurs in lesions 5 cm or greater in diameter. Besides hemorrhage, the other main worrisome complication of HCA is malignant transformation to HCC, with a frequency of 4% to 9% in surgical series, found in lesions over 4 cm in diameter.
The majority, 70% to 80%, of HCAs are solitary. Multiple lesions are often seen with anabolic steroid use and glycogen storage diseases. An entity called liver adenomatosis has been described consisting of greater than 10 adenomas in patients without hormonal risk factors or glycogen storage disease. However, this may not warrant a separate classification, since many of the cases described have been in women on oral contraceptives and other than the number of lesions, there is no difference in imaging or pathology.
On sectioning, HCAs are typically well circumscribed round or oval masses 5 to 15 cm in diameter with a variegated appearance from areas of hemorrhage, necrosis, and infarction. They are usually unencapsulated, but a thin fibrous capsule is sometimes present. Histologically, they are composed of a trabecular pattern of normal appearing hepatocytes that may have increased cytoplasmic fat and glycogen. Thin-walled arteries supply the parenchyma but without other elements of portal triads including bile ducts or significant connective tissue support, which may predispose to hemorrhage. Degenerative changes are commonly seen on histology, including infarction, hemorrhage, peliosis, and sinusoidal dilatation.
MR imaging features
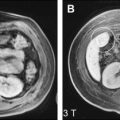
The appearance of HCA on MR imaging is quite variable, reflecting the various gross pathologic features. Focal areas of high T1 signal are present in 45% to 77% of HCAs, which correspond to steatosis, hemorrhage, or peliosis on pathologic correlation ( Fig. 2 ). With steatosis, signal intensity loss on chemical shift imaging is seen. On T2-weighted sequences, 47% to 74% of HCAs are predominantly hyperintense with only 4% to 10% hypointense. Heterogeneity is present on T1- or T2-weighted sequences in 51% to 94%, correlating pathologically with hemorrhagic necrosis and peliosis. A capsule is seen on MR imaging in 17% to 31% of cases, which demonstrates low T1 and variable T2 signal intensity.
During the administration of intravenous gadolinium, 96% of HCAs demonstrate marked arterial enhancement ( Fig. 3 ). Signal intensity in the portal venous and equilibrium phases is more varied and HCAs may be hyperintense, isointense, or hypointense although the majority of HCAs appear isointense in the equilibrium phase. Contrast enhancement may be heterogeneous or homogeneous. Kupffer cells are reduced in number and function so adenomas rarely show uptake of SPIO particles.
Differential diagnosis
FNH, HCC, hypervascular metastases, as well as HCA should be considered in the differential diagnosis of hypervascular hepatic lesions. In a study by Arrivé and colleagues, 88% of adenomas had heterogeneous signal, T1 hyperintensity or a peripheral rim, which distinguish them from FNH. Conversely, the demonstration of a central scar-like area of fibrosis is unusual in HCA. However, the MR imaging features of FNH without a central scar are similar to homogeneous HCA without hemorrhage, fat or necrosis, particularly seen in smaller lesions. Delayed imaging with gadobenate dimeglumine may be helpful in these cases. Fibrolamellar carcinoma is more common in men than HCA and may be distinguished by a central scar, lobulated margins, and malignant features. There is tremendous overlap in the features of HCA and HCC. Biopsy is often performed for HCAs not requiring surgical resection. MR elastography, which measures the stiffness of tissue, may help differentiate benign tumors, including HCA, from malignant tumors since benign hepatic tumors demonstrate lower shear stiffness, based on preliminary results. Hypervascular metastases are usually multiple, markedly hyperintense on T2-weighted sequences, and rarely contain fat and hemorrhage.
Biliary cystadenoma and cystadenocarcinoma
Clinical and pathologic features
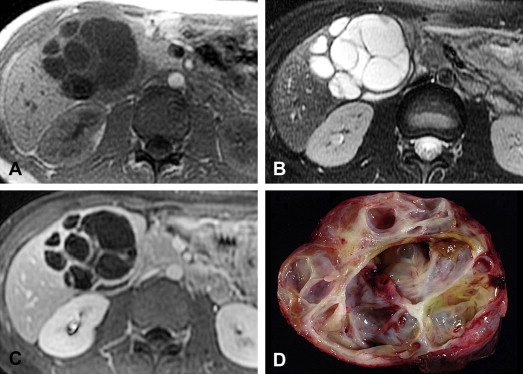
Biliary cystadenomas are rare cystic neoplasms, accounting for less than 5% of intrahepatic cysts of bile duct origin in a surgical series. Other biliary derived cysts include simple hepatic cysts, polycystic liver disease, choledochal cysts, and biliary hamartomata. Biliary cystadenocarcinomas are the malignant counterpart of cystadenomas, and are discussed together with cystadenomas because of overlapping pathologic and imaging features. Biliary cystadenomas are most commonly found in middle-aged women, with an average age of 38 to 45 years and female preponderance of 93% to 96%. Biliary cystadenocarcinomas occur on average at a slightly older age, 56 years, and are relatively more common in men than women compared with cystadenomas (62% of cystadenocarcinomas are found in women). Clinical symptoms are nonspecific, including abdominal pain and palpable mass. Jaundice can be seen when there is biliary obstruction.
The majority of biliary cystadenomas and cystadenocarcinomas are intrahepatic, but occur occasionally in the extrahepatic bile ducts and gallbladder. They are encapsulated, multilocular cystic masses with a smooth outer contour. The cyst contents vary and may be clear, mucinous, bilious, hemorrhagic, or mixed fluid. Biliary cystadenocarcinomas are more likely than cystadenomas to demonstrate papillary excrescences and polypoid masses in the cyst cavities.
Benign columnar or cuboidal epithelial cells line the cyst locules of cystadenomas. Similar benign appearing epithelium is found in addition to malignant epithelial cells in over 90% of biliary cystadenocarcinomas, supporting the theory of a progression between the two. A layer of mesenchymal tissue that resembles ovarian stroma usually surrounds the epithelium. The ovarian-like stroma is only seen in women, and may confer a better prognosis in cases of cystadenocarcinoma.
MR imaging features
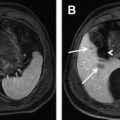
Cystadenomas and cystadenocarcinomas are multilocular cystic masses on imaging ( Fig. 4 ). MR imaging allows characterization of the cyst contents, although the signal varies with protein concentration and age of hemorrhage. In general, compared with muscle, mucinous fluid is isointense on T1-weighted sequences and hyperintense on T2-weighted sequences, serous fluid has low T1 and high T2 signal intensity, and hemorrhage can be identified by high T1 signal intensity or fluid-fluid levels. A low T2 signal intensity outer capsule may be seen, likely from hemorrhage within the wall. With contrast administration, there is enhancement of the cyst wall and septa. Although the imaging findings overlap, the presence of enhancing mural nodules is suggestive of biliary cystadenocarcinoma, and their absence favors biliary cystadenoma. No imaging finding correlates with the presence of ovarian-like stroma.
Magnetic resonance cholangiopancreatography (MRCP) can help define the relationship of the cyst to the bile ducts. Dilatation of the peripheral bile ducts may be seen from obstruction. Rarely, biliary cystadenomas or cystadenocarcinomas may communicate with or prolapse into a bile duct.
Differential diagnosis
The differential diagnosis of a hepatic complex cystic mass is broad. Infectious causes, including pyogenic abscess, amebic abscess, and hydatid cysts are a main consideration. Clinical and laboratory data, including amebic and echinococcal serologies, can help distinguish these. Helpful MR imaging findings include low signal intensity, nonenhancing walls of the daughter cysts, or nonenhancing membranes within a hydatid cyst. Pyogenic and amebic abscesses are not encapsulated, have ill-defined enhancing margins, and often have a rim of edema between central areas of necrosis and adjacent normal liver. Cystic hepatic neoplasms, including mesenchymal hamartoma and undifferentiated embryonal cell sarcoma, are usually found in children. Cystic metastases are often multiple and there is a clinical history of known primary. Hepatocellular carcinoma may have a cystic appearance when there is significant necrosis after treatment.
Nonepithelial
Cavernous hemangioma
Clinical and pathologic features
Cavernous hemangioma is the most common benign hepatic tumor with a reported prevalence of 1% up to 20%. It can occur at any age and demonstrates a female predominance, with a female-to-male ratio of 2:1 to 5:1. The vast majority are incidentally found. Patients with large lesions may present with abdominal pain or symptoms from mass effect on adjacent structures. Rare complications include intratumoral hemorrhage, rupture with hemoperitoneum, and unusual hematologic manifestations such as erythrocytosis from secretion of erythropoietin and Kasabach-Merritt syndrome, which consists of a consumptive coagulopathy, thrombocytopenia, and hemolytic anemia.
Cavernous hemangiomas are well-circumscribed and vary from less than 1 cm to over 30 cm in size. Ten to twenty percent are multiple and there is a reported association with focal nodular hyperplasia. Microscopically, a honeycomb appearance is generated by numerous blood filled spaces, each lined by a single layer of flat endothelial cells in contrast to hepatic peliosis, which is characterized by blood-filled spaces without an endothelial lining. They can be quite heterogeneous, containing areas of recent or organized thrombus, fibrosis, and, rarely, calcification. Fibrosis is typically central but can involve the entire lesion, referred to as a sclerosed or hyalinized hemangioma.
MR imaging features
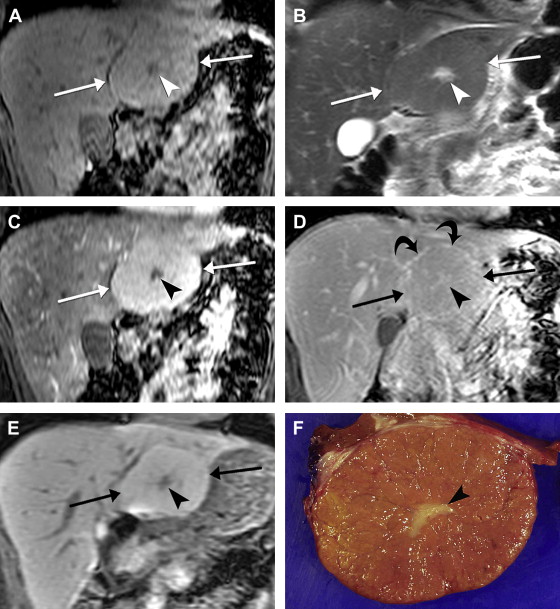
On imaging, hepatic cavernous hemangiomas have smooth well-defined margins and are round or lobular in shape. They are hypointense on T1-weighted sequences except for regions of hyperintensity in the rare occasion of hemorrhage. Hemangiomas have high T2 signal intensity approaching that of cerebrospinal fluid and show even higher signal intensity on long TE T2-weighted sequences. They may not be completely homogeneous, with nodules or septa of low T2 signal intensity in 80% of cases, corresponding to fibrosis on pathology. Additionally, many giant hemangiomas, variably defined as greater than 4 cm up to greater than 12 cm in diameter, contain a central cleft of cystic degeneration or liquefaction that compared with the remainder of the lesion is hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences ( Fig. 5 ).
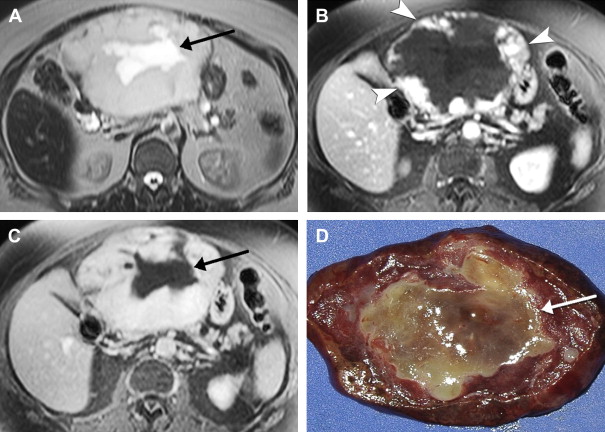
During the administration of intravenous gadolinium contrast agents, a pattern of early peripheral discontinuous nodular enhancement with progressive centripetal complete or incomplete filling-in has 84% sensitivity, 100% specificity, and 95% accuracy for the diagnosis of hemangioma. The signal intensity of the enhancing areas parallels aortic enhancement (see Fig. 5 B and C). The fibrous septa and central clefts of large hemangiomas do not enhance (see Fig. 5 C). Another frequent enhancement pattern is early homogeneous hyperenhancement, which is often called flash-filling. In a study of 154 hemangiomas by Semelka and colleagues, 23% displayed that pattern; all were less than 1.5 cm in diameter.
Completely sclerosed hemangiomas demonstrate extensive fibrosis, resulting in atypical imaging findings. Volume loss from fibrosis may cause adjacent retraction of the hepatic capsule. T2 signal may be less hyperintense and there may be a lack of early enhancement with delayed slight peripheral enhancement. Increased fibrosis with volume loss and capsular retraction can also be seen in hemangiomas in the setting of progressive cirrhosis.
Differential diagnosis
There is no differential diagnosis for cavernous hemangiomas that demonstrate a classic pattern of contrast enhancement. The imaging features of lesions with rapid homogeneous enhancement overlap with other hypervascular liver tumors, including HCC, HCA, and hypervascular metastases. One helpful finding in differentiating a flash-filling hemangioma from a hypervascular metastasis is hyperintensity of a hemangioma on the delayed phase. Metastases often demonstrate heterogeneous or peripheral washout.
Angiomyolipoma
Clinical and pathologic features
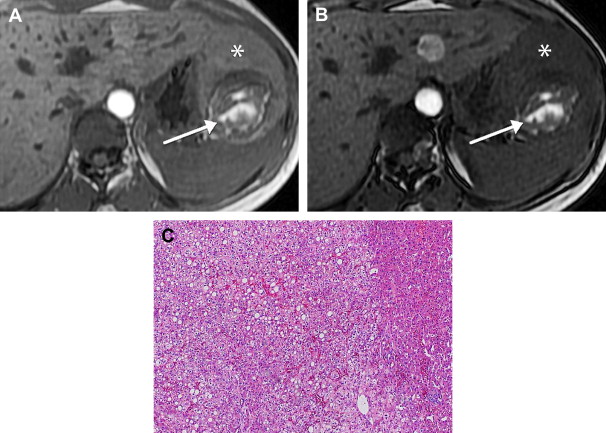
Angiomyolipoma is a benign tumor composed of smooth muscle, fat, and thick-walled blood vessels. It rarely occurs in the liver, with over 200 cases reported in medical literature. The average age of presentation is 50 years (range 10 to 79) with a female predominance. The majority are sporadic, although 6% to 10% occur in patients with tuberous sclerosis. Conversely, 13% of patients with tuberous sclerosis have hepatic angiomyolipomas, which are usually multiple and associated with multiple bilateral renal angiomyolipomas. Patients either have nonspecific symptoms such as abdominal discomfort or mass or the lesion is an incidental finding, although intraperitoneal rupture and malignant transformation rarely occur.
Angiomyolipomas are well-circumscribed nonencapsulated round or ovoid masses that vary in size from less than 1 cm to 36 cm in diameter. The cut surface is yellow to tan in color and focal areas of hemorrhage or necrosis may be present. Microscopic findings are diverse since the relative proportion of smooth muscle cells, mature adipose tissue, and blood vessels varies widely. The myoid component usually predominates and consists of spindle cells, epithelioid cells, and intermediate ovoid or short spindle cells. The fat component varies from scattered cells involving less than 10% of the tumor in 30% of cases to greater than 70% in 10% of cases. The vascular component includes both a rich capillary network in addition to multiple tortuous thick-walled vessels with occasional calcification.
MR imaging features
Hepatic angiomyolipomas demonstrate a spectrum of appearances on MR imaging, reflecting their varied histologic composition. They may be hyperintense, hypointense, or heterogeneous with hyperintense and hypointense areas on T1-weighted sequences, depending on the amount and distribution of fat. Areas of macroscopic fat demonstrate peripheral low signal (etching artifact or India ink artifact) on out-of-phase sequences and diffusely decreased signal intensity with fat-suppression sequences ( Fig. 6 ). Areas of microscopic fat demonstrate diffuse loss of signal on out of phase sequences. Fat signal intensity may not be present in lesions with minimal pathologic fat composition. On T2-weighted sequences, hepatic angiomyolipomas are homogeneously or heterogeneously hyperintense. With gadolinium, most hepatic angiomyolipomas demonstrate hyperenhancement during the arterial phase and may be hyperintense, isointense, or hypointense during the portal venous and delayed phases.
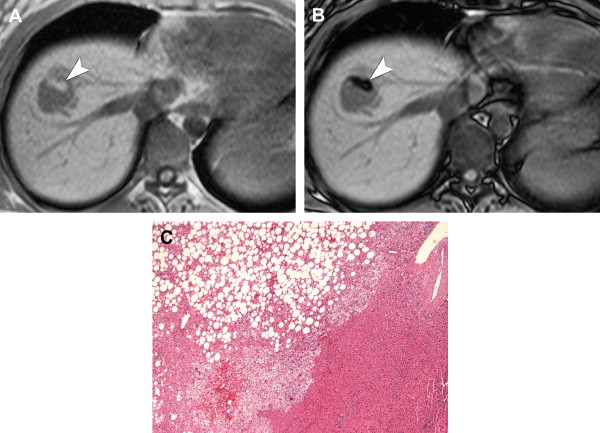
Differential diagnosis
HCA, HCC, myelolipoma, and fat-containing metastases, such as liposarcoma and malignant teratoma, can contain fat and soft tissue components and their MR imaging appearance may be difficult to distinguish from angiomyolipomas.
Solitary fibrous tumor
Clinical and pathologic features
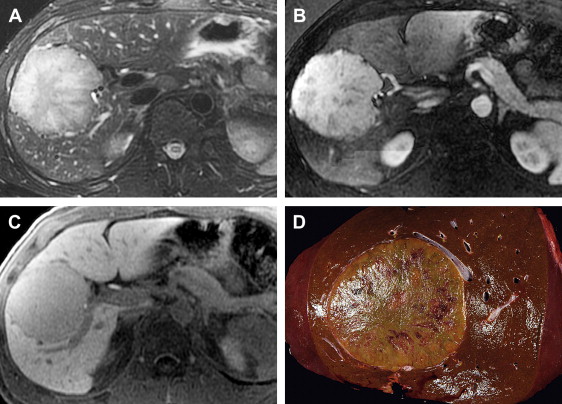
SFT is a rare hepatic neoplasm that is similar to tumors arising in the pleura, mediastinum, and other sites. It occurs in adults, with a mean age of 57 years and a 2:1 female-to-male ratio. Clinical presentation varies from asymptomatic to abdominal discomfort or fullness due to mass effect.
SFT is typically a large well-circumscribed intrahepatic or pedunculated firm mass with a nodular or smooth surface. When sectioned, these tumors are gray to white in color with a whorled appearance and may contain central hemorrhage or necrosis. Bundles of spindle cells with either a haphazard arrangement or a storiform pattern are present microscopically. The majority of SFTs are benign in biologic behavior but malignant transformation may occur. The true incidence of malignant transformation is not known owing to the low number of reported tumors. With slightly greater than 50 tumors appearing in the literature to date, to the authors’ knowledge there is only one reported case of a primary hepatic SFT with distant metastases.
MR imaging features
When evaluated by MR imaging, SFT of the liver is characteristically hypointense on T1-weighted sequences, variably hypointense or hyperintense on T2-weighted sequences and demonstrates heterogeneous enhancement following the administration of intravenous contrast. Contrast enhancement may be avid and progressive, increasing through the arterial and venous phases resulting in increased enhancement in the delayed phase ( Fig. 7 ). The marked enhancement of some tumors is thought to be due to prominent vascularity.
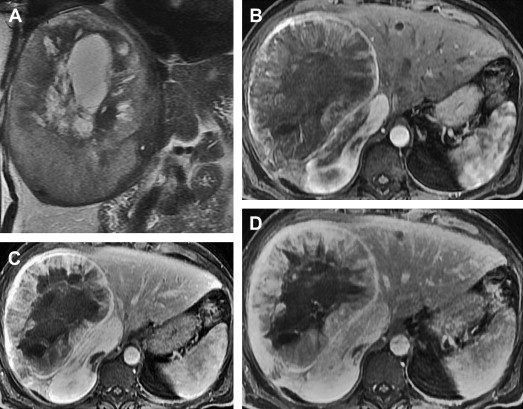
Differential diagnosis
The imaging findings of a large, solitary, well-demarcated, heterogeneous mass are largely nonspecific. Progressive delayed enhancement can be seen with fibrotic lesions, including ICC, the sclerosing type of HCC and epithelioid hemangioendothelioma in addition to SFT. Vascular lesions such as cavernous hemangioma and angiosarcoma also demonstrate progressive enhancement, although the typical early peripheral discontinuous nodular enhancement pattern of a hemangioma distinguishes it from SFT.
Malignant tumors
Epithelial
Hepatocellular carcinoma and precursor lesions
Clinical and pathologic features
HCC is the most common primary liver malignancy in adults. It is the fifth most common cancer in the world, although there is striking geographic variation depending on the prevalence of major risk factors. Hepatitis B virus infection and aflatoxin B1 contribute to the high incidence in Africa and parts of Asia, whereas hepatitis C virus infection is the main risk factor in Japan. The incidence of HCC in the United States has more than doubled in the past two decades, primarily related to an epidemic of hepatitis C virus infection from the 1960s to the 1980s. Other predisposing factors include cirrhosis of any cause, such as heavy alcohol consumption, hemachromatosis, hereditary tyrosinemia, and alpha-1-antitrypsin disease. Obesity and diabetes are also related to HCC through nonalcoholic fatty liver disease. Between 15% and 50% of patients in the United States have no known risk factor.
The incidence of HCC increases with age, with a mean age of diagnosis of 65 years in the United States. In countries where hepatitis B virus is endemic, HCC occurs at an earlier age, often before 40. Three quarters of patients are male, which may relate to higher rates of known risk factors and androgen receptors often present on the tumors. Clinical symptoms vary widely. Most patients present with abdominal pain, weight loss, and hepatomegaly, often with a palpable mass. Decompensated cirrhosis may be the only indication of HCC, likely from neoplastic portal or hepatic vein thrombosis. Rarely, HCC can cause obstructive jaundice, spontaneous rupture with hemoperitoneum, or paraneoplastic syndromes, including hypertrophic osteoarthropathy. Serum alpha-fetoprotein is reportedly elevated in 70% to 90% of patients; however, it is usually normal or minimally elevated in patients with small HCCs (defined as >2 cm in diameter). The sensitivity and specificity of serum alpha-fetoprotein as a screening test vary widely depending on the cutoff value (<20 ng/mL is most commonly used) in addition to other factors, and higher quality studies are needed to determine its utility.
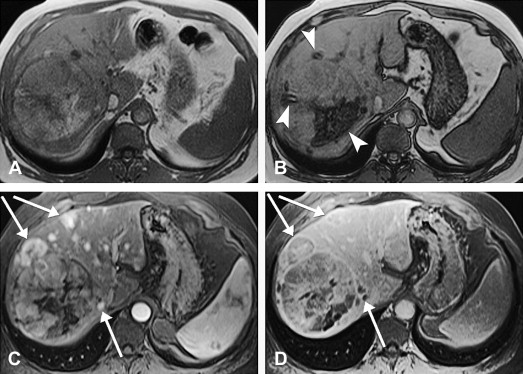
HCC may arise from a single cell or group of hepatocytes or may develop in a stepwise pattern in cases of cirrhosis from a regenerative nodule through a spectrum of low-grade and high-grade dysplastic nodules. A regenerative nodule is a localized proliferation of hepatocytes and support stroma with normal blood supply and no atypia. Low-grade and high-grade dysplastic nodules demonstrate increasing nuclear to cytoplasmic ratios, nuclear atypia, distortion of the normal plate architecture, and increasing arterial supply. These changes are more advanced in well-differentiated HCC, which are also associated with mitotic figures and invasion of the stroma or portal tracts. There are several histologic growth patterns of HCC, with a trabecular pattern being most common ( Fig. 8 A ). The fibrolamellar pattern, which has abundant stroma, has distinct clinical and pathologic features and is discussed separately.
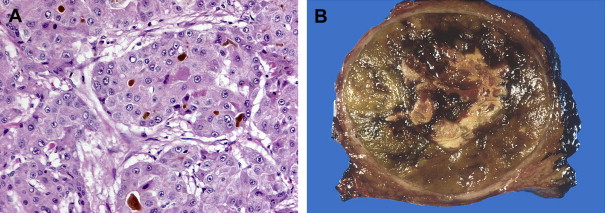
A single mass with or without satellite nodules is the most common gross appearance of HCC. It also may arise as multiple discrete nodules throughout the liver or more rarely diffuse infiltration. Large tumors are often heterogeneous and a mosaic appearance may be seen from areas of steatosis, hemorrhage, cholestasis, fibrosis, and necrosis (see Fig. 8 B). They are soft tumors because of a lack of desmoplasia except for fibrolamellar and rare scirrhous variants. A fibrous capsule is common in lesions over 1.5 cm in diameter. Vascular invasion is found in close to three quarters, usually involving the portal or hepatic veins. Bile duct invasion is much more infrequent, seen in about 3%. Intrahepatic, lung, regional lymph nodes, bone, and adrenal gland are the most common sites of metastatic disease.
MR imaging features
MR imaging has the highest combination of sensitivity and specificity for HCC compared with other imaging modalities, at 81% and 85%, respectively. However, sensitivity is less for smaller tumors that may be similar in appearance to cirrhotic nodules. Regenerative nodules and low-grade dysplastic nodules demonstrate variable T1 signal intensity, low T2 signal intensity and are isointense or slightly hypointense with intravenous gadolinium enhancement. High-grade dysplastic nodules are usually less than 2 cm in diameter and can display slightly higher T2 signal intensity and arterial phase enhancement with portal venous phase washout, similar to small HCC. Small HCC are usually well differentiated and demonstrate variable T1 signal intensity, slightly high T2 signal intensity, and homogeneous intense arterial phase enhancement with portal venous phase washout. Small HCC also may have a nodule-in-a-nodule appearance, indicating that it is arising within a larger regenerative or dysplastic nodule.
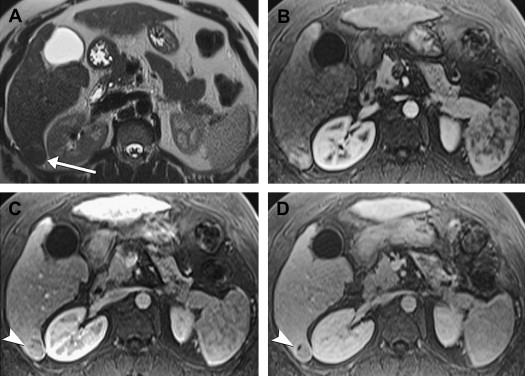
The appearance of large HCCs is quite variable. The most common pattern is hypointensity on T1-weighted sequences, hyperintensity on T2-weighted sequences and diffuse heterogeneous immediate enhancement ( Fig. 9 ). Hyperintense T1 signal can be seen, from intratumoral fat, copper, or glycogen, and is more common in low-grade tumors. A mosaic pattern of varying signal intensities on unenhanced and enhanced sequences is frequent, reflecting the heterogeneous pathologic appearance. A tumor capsule, more common in larger tumors, usually demonstrates low T1 and T2 signal intensity and delayed enhancement. In capsules over 4 mm in thickness, inner low T2 and outer high T2 signal intensity layers may be seen from layers of inner fibrous tissue and outer compressed vessels and bile ducts. Evidence of invasiveness is frequent in larger tumors, including extracapsular extension, satellite nodules ( Fig. 10 ), vascular invasion, and metastatic disease. Tumor thrombus can be distinguished from bland thrombus by expansion of the lumen and contiguity to and similar imaging characteristics with the primary tumor, including on T2, postcontrast and diffusion-weighted sequences.
In addition to gadolinium chelates, several other intravenous contrast agents have been studied in the diagnosis of HCC. After the administration of SPIO particles, HCC is typically hyperintense on T2- and T2-weighted sequences although some well-differentiated tumors may demonstrate uptake and be isointense or hypointense. To improve sensitivity, some investigators advocate a double-contrast protocol of SPIO particles and a gadolinium chelate because of their synergistic effects. Combined extracellular and hepatocellular agents, including gadoxetic acid, may also improve sensitivity. Larger prospective studies are needed to determine the best screening method.
Differential diagnosis
In patients with cirrhosis, arterial enhancing lesions are not uncommon and, in addition to HCC, they may represent dysplastic nodules or transient arterial enhancement from arterioportal shunts or obstructed distal parenchymal portal veins. In one study, 93% of arterial enhancing lesions less than or equal to 2 cm in diameter and not seen on other phases of enhancement or unenhanced sequences were nonneoplastic. Confluent fibrosis can appear mass-like and have hyperintense T2 signal intensity, although it typically is wedge-shaped, demonstrates delayed enhancement, may be associated with capsular retraction, and is commonly centrally located, in the anterior and medial segments of the liver.
In patients without cirrhosis, the differential diagnosis of small HCCs includes other arterial enhancing masses such as flash-filling hemangiomas, FNH, HCA, and hypervascular metastasis. The differential of a large, encapsulated, heterogeneous mass includes HCC, HCA, SFT, and hepatic sarcomas.
Fibrolamellar carcinoma
Clinical and pathologic features
Fibrolamellar carcinoma is a distinct variant of HCC seen in young patients usually without previous liver disease. It has an equal gender distribution with a mean age of presentation of 23 years. Presenting symptoms range from abdominal pain, hepatomegaly, and palpable mass to, more rarely, gynecomastia or venous thrombosis. Serum alpha-fetoprotein levels are generally not elevated in contrast to patients with HCC; however, mild elevation may occur in approximately 10% of patients.
The gross appearance of fibrolamellar carcinoma is usually a nonencapsulated but well-demarcated solitary mass. Less frequent morphologic appearances include a mass with peripheral satellite lesions, bilobed mass, or diffuse multifocal disease. Two thirds occur in the left lobe. Fibrolamellar carcinoma is characterized histologically by prominent fibrous lamellae ( Fig. 11 ) supporting groups of tumor cells that contain a coarse eosinophilic granular cytoplasm attributable to the presence of abundant mitochondria. The fibrous tissue may coalesce into a central scar, which commonly contains calcification.