Table 33.3 shows the National Wilms’ Tumour Study Group (NWTS) staging system for Wilms’ tumour. In 4–8% of cases tumours are bilateral (stage V).
Table 33.3 National Wilms’ Tumour Study Group (NWTS) staging system
| Stage | Clinicopathological features |
|---|---|
| I | Tumour confined to within renal capsule, completely excised |
| II | Tumour invading outside renal capsule, completely excised |
| III | Residual abdominal tumour – positive margins, tumour rupture, involved nodes |
| IV | Haematogenous metastases |
| V | Bilateral disease |
The current long-term survival rate for Wilms’ tumour is in excess of 80%. Treatment is aimed at maintaining this high survival rate while attempting to reduce the long-term side effects of therapy. There is a major disparity between approaches adopted in Europe and North America. The North American series of NWTS, now COG protocols, employ immediate nephrectomy with staging and post-surgical adjuvant therapy based on histological examination of the primary tumour. Wilms’ tumour histology is referred to as either favourable (FH) or unfavourable (UH) because of the presence of anaplasia.
Postoperative chemotherapy is given using the drugs vincristine, actinomycin-D and doxorubicin, the number of drugs and duration depending upon the staging.
In Europe, the series of International Society of Paediatric Oncology (SIOP) studies have been based on preoperative chemotherapy to ‘downstage’ the primary, reducing the surgical morbidity, particularly the number who have tumour rupture at surgery, and the number who require flank radiotherapy. The most recent study is the SIOP 2001 study into which patients from the UK are entered. All patients receive preoperative chemotherapy with actinomycin-D and vincristine, with delayed nephrectomy after 6 weeks of preoperative chemotherapy. Postoperative adjuvant therapy is based on subsequent pathological staging and allocation of risk status (good-risk versus intermediate-risk versus poor-risk histology). For intermediate-risk patients, there is a randomization to receive or not receive doxorubicin. The purpose of this is to determine whether doxorubicin can be omitted, thus reducing the risk of late cardiac sequelae.
Postoperative flank radiotherapy is employed for stage III patients, i.e. those with incompletely resected primary tumours, pre- or perioperative tumour rupture, or histologically involved lymph nodes. The technique employs anterior and posterior opposed fields. The CTV includes the preoperative extent of tumour and kidney, following preoperative chemotherapy with a margin of 1.0 cm. Fields extend across the midline in order to irradiate homogeneously the full width of the vertebral body in order to minimize the risk of kyphoscoliosis (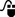 ). For patients with intermediate-risk histology, the dose is 14.4 Gy in eight fractions of 1.8 Gy daily, and for high-risk histology, 25.2 Gy in 14 fractions of 1.8 Gy. There is a boost to macroscopic disease or involved nodes: 10.8 Gy in six fractions of 1.8 Gy.
). For patients with intermediate-risk histology, the dose is 14.4 Gy in eight fractions of 1.8 Gy daily, and for high-risk histology, 25.2 Gy in 14 fractions of 1.8 Gy. There is a boost to macroscopic disease or involved nodes: 10.8 Gy in six fractions of 1.8 Gy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree