Introduction
The abdominal aorta is the continuation of the thoracic aorta and the major conduit artery distributing blood to the abdominal organs and then to the lower extremities. Pathologic processes that affect the abdominal aorta, in order of decreasing incidence, are: atherosclerosis (mostly nonhemodynamic significant plaques), abdominal aortic aneurysm formation, various forms of vasculitis, genetically based degenerative disease of the aortic wall, and the extension of proximal thoracic aortic dissections.
Anatomy
The abdominal aorta extends from the level of the diaphragm to the common iliac artery bifurcation (i.e., roughly at the level of the umbilicus). It is an elastic artery and elastin fibers give it mechanical strength. Oxygen is supplied to the arterial wall from the aortic lumen and by the small arterioles that comprise the vasa vasorum of the adventitia. Because of this dual supply and the limits of oxygen diffusion, a region of relatively poor perfusion lies approximately at the middle third of the media. This portion of the aortic wall is prone to the development of cystic medial degeneration (necrosis). The abdominal aortic wall is less prone to cystic medial degeneration than the thoracic aortic wall. Genetic defects in the FBN1 gene also make the media susceptible to dissections as seen in Marfan or Ehlers-Danlos syndromes. The presence of cystic medial degeneration increases the risk of thoracic aortic wall dissection and of intraluminal hematoma formation.
The major branches of the abdominal aorta include the celiac axis, the superior mesenteric artery, the renal arteries, the inferior mesenteric artery, the gonadal arteries, and the lumbar arteries. The celiac axis has an anterior origin, most often near the midline at approximately the level of the 12th thoracic vertebral body and the first lumbar artery. The superior mesenteric artery originates at approximately the level of the first and second lumbar vertebral arteries and is slightly to the left. The renal arteries have their origins just inferior to the superior mesenteric artery and are located to the side of the aorta. The inferior mesenteric artery has its origin just above the level of the aortic bifurcation and oriented slightly to the left of the midline. Both gonadal arteries arise from the aorta between the superior and inferior mesenteric arteries. The paired lumbar arteries are located on both sides of the posterior aspect of the aorta. There are normally four pairs of lumbar branches, the lower one possibly arising from iliac artery branches.
Normal Sizes
The abdominal aorta gradually tapers as it courses through the abdomen. The average diameter of the aorta is smaller in women than in men. Normal values vary with age, progressively increasing from childhood into adulthood. These size ranges are shown in Table 24.1 . By adulthood, the average diameter of the abdominal aorta is approximately 27 mm at the diaphragm, tapering to approximately 21 mm at the iliac bifurcation. For women, diameters are smaller by approximately 3 to 5 mm.
| Complication | Mechanism | Comment |
|---|---|---|
| Rupture | Mechanical failure and wall breakdown as size increases | Lags by 10 years in women |
| Pain (back pain or lower abdominal) | Possibly due to inflammatory component | More likely in adults 50 years or younger |
| Hydronephrosis | Compression of the ureters | Very large aneurysms |
| Distal embolization | Associated with mural thrombus | Uncommon |
| Acute thrombosis | Associated with atherosclerosis and stenosis | Rare |
Normal Doppler Velocity Profiles
Peak systolic velocities in the abdominal aorta average 110 cm/s in a population with an average age of 12 years. With aging, the average velocity decreases, ranging from 70 to 100 cm/s. The aortic blood flow patterns change with distance from the diaphragm. Near the diaphragm, the waveforms exhibit a low-resistance blood flow pattern during diastole ( Fig. 24.1A ). This diastolic component is a reflection of the low-resistance vascular beds located slightly lower in the abdominal aorta: the liver, spleen (from the celiac axis), and the kidneys. These organs have low-resistance blood flow patterns, in essence monophasic (see Chapter 23 ). Below the renal arteries, the aortic Doppler tracings will show a typical peripheral waveform appearance (see Fig. 24.1B ). This triphasic appearance with early diastolic reversal is caused by the high impedance of the lower extremity arteries. The low-resistance component of blood flow to the kidneys, spleen, and liver no longer contribute to decreasing the high outflow resistance/impedance seen in the distal abdominal aorta. The inferior mesenteric artery has a minor contribution to the waveform appearance, if any, given that it is small and has a high resistance pattern in the fasting state.

Blood flow patterns in the proximal abdominal aorta will therefore be affected, to a certain extent, by the state of the renal arteries and the celiac axis. There have not been any systematic studies of the variations of adult abdominal aorta Doppler tracings linked to pathologic changes in these organs. For example, no specific studies have looked at the Doppler aortic waveforms in the presence of chronic renal failure.
- •
The abdominal aorta is an elastic artery. Poor perfusion to the media or genetic defects in the collagen/elastin constituents make it prone to arterial wall dissections.
- •
The normal abdominal aorta tapers uniformly from the diaphragm to the origin of the iliac arteries and should be less than approximately 27 mm in men and 23 mm in women.
- •
The Doppler waveform appearance of the abdominal aorta depends on the level where it is sampled: low resistance above the renal arteries and high resistance below. Peak systolic velocities should be 100 cm/s or less.
Pathologic States
Atherosclerosis and occlusive arterial disease
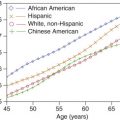
The atherosclerotic process affects the aortic wall relatively early in life and is prevalent by the late 50s in men ( Fig. 24.2 ) with a lag of about 10 years in women. Most autopsy studies of the distribution of arterial disease show that the abdominal aorta is one of the first affected arteries along with the thoracic aorta. Involvement of the abdominal aorta alone is uncommon in the spectrum of atherosclerotic disease. Historically, isolated symptomatic aortic lesions were described in relatively young patients (mean age of 55 years) and more often in women, with smoking being a significant risk factor. Recent studies suggest that this gender bias might not be the case. Clinically, high-grade lesions develop slowly, are located below the renal arteries, and are associated with the concurrent development of collateral channels. However, acute aortic occlusions can occur rapidly and acutely compromise lower extremity blood flow. Progressive aortic occlusive narrowing tends to be accompanied by slowly increasing lower extremity symptoms. Symptoms can be very restrictive, and the patients adapt by decreasing their walking distance. Ultimately, symptoms progress to pain at rest and may result in impotence in men. This condition is referred to as Leriche syndrome.

Total occlusion of the abdominal aorta is rare and tends to occur in the lower portion of the abdominal aorta with thrombus spreading upward (cephalad). Although the occlusive process and the associated thrombus formation may extend to the level of the renal arteries, they generally stay patent and rarely occlude. The superior mesenteric artery is rarely affected and serves as a source of collateral blood flow to the lower extremities via collaterals to internal iliac artery branches. The right and left inferior epigastric arteries are also major collateral pathways.
Doppler imaging at the level of the common femoral arteries can suggest the diagnosis of either occlusion or severe stenosis of the abdominal aorta ( Fig. 24.3 ). This indirect approach will not fully document the type and extent of aortic disease or isolated involvement of both common iliac arteries. Doppler tracings will typically show a monophasic (tardus-parvus) pattern common to any severe proximal arterial occlusion with peak velocities of 45 cm/s or less. With acute occlusions, diastolic velocities may also be depressed prior to the development of collaterals. The diastolic component of the waveform will become more prominent as collateral pathways develop and the relative magnitude of diastolic blood flow is a reflection of the ability of the peripheral circulation to recruit collaterals.

Doppler ultrasound can confirm the presence of an aortic occlusion by the absence of blood flow in the involved aortic segment. No large series has evaluated the accuracy of this sonographic evaluation. Grading of stenosis severity in the aorta by Doppler criteria has also not been extensively studied because of the rarity of isolated abdominal aortic stenosis. The diagnostic criteria currently used to grade aortic stenosis severity were adapted from the peak systolic velocity ratio criteria used for the determination of lower extremity arterial stenosis ( Fig. 24.4A and B ). As discussed in the chapter dealing with the lower extremity arteries (see Chapter 15 ), the Doppler peak systolic velocity ratio is determined by performing a Doppler velocity measurement at the site of maximal blood flow velocity and then dividing this peak systolic velocity by a normal aortic peak systolic velocity. This normal velocity is typically measured in a contiguous segment of the aorta located above the stenosis. In cases of diffuse disease or in the absence of a good acoustic window, distal peak systolic velocities can be used to determine the peak systolic velocity ratio. This can be used only in the absence of turbulent or disturbed flow related to a distal stenosis. As in the lower extremity, a velocity ratio of 2 or greater is used to indicate the presence of at least 50% diameter stenosis.

Abdominal Aortic Aneurysm
Types of aneurysms
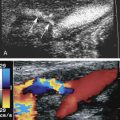
Abdominal aneurysms are more common than thoracic aneurysms ( Fig. 24.5 ). Infrarenal aneurysms are also more common than suprarenal aneurysms (see Fig. 24.5 ). There are two principal types of aneurysms: fusiform and saccular ( Fig. 24.6 ). By far the more common form of aneurysm is the fusiform type. Fusiform aneurysms are associated with risk factors such as high blood pressure and smoking, whereas saccular aneurysms are associated with inflammation and infection.


Although fusiform aneurysms (see Fig. 24.6 ) exhibit varied appearance and degrees of eccentricity ( Fig. 24.7A and B ), all three layers of the aortic wall are intact. On the other hand, saccular aneurysms demonstrate discontinuity in the layers of the aortic wall ( Figs. 24.6, 24.8, and 24.9 ). Fusiform aneurysms typically form a symmetric and concentric bulge but can also have an eccentric appearance ( Fig. 24.10 ).





Saccular aneurysms are, by their nature, very eccentric because not all layers of the artery wall are intact. They are much less common than fusiform aneurysms, making up about 1% of all aortic aneurysms. Most saccular aneurysms occur in the thoracic aorta and much less often in the abdominal aorta. There are various possible etiologies: trauma, infection, vasculitis, and spontaneous dissections into the aortic wall. Although traumatic pseudoaneurysms typically rupture through all three layers of the aortic wall, they are often called saccular aneurysms because of their appearance. By far the most common location for a traumatic saccular aneurysm is the thoracic aorta. Prior instrumentation, such as placement of a cannula in the aortic wall, can lead to the formation of a saccular aneurysm.
The second type of saccular aneurysm is associated with various infections and is often called mycotic. Mycotic aneurysms develop because of an acute infection of the aortic wall, usually from the Streptococcus and Staphylococcus as well as the nontyphoid Salmonella species, although any organism can be involved. Interestingly, the term mycotic implies that a fungus is the source of the infection, but fungal mycotic aneurysms are rare. Candida is the most common fungal organism seen in mycotic aneurysms. Saccular and mycotic aneurysms are similar in appearance, and they can be distinguished by positive bacterial or fungal cultures and the patient’s clinical presentation.
The third type of saccular aneurysm is associated with vasculitis. The inflammatory process begins within the vasa vasorum of the adventitia and spreads to cause fibrosis and degeneration of the media. Breakdown of the media produces a saccular aneurysm. This is typical of Behçet syndrome.
The fourth type of saccular aneurysm occurs when a penetrating ulcer ruptures into the media of the aorta. This leads to a contained leak into the wall and eccentric pressure promotes the formation of the aneurysm. The major difference with a pseudoaneurysm is that the adventitia, and very commonly a part of the media, remain intact in a saccular aneurysm. All three layers are ruptured in the case of a pseudoaneurysm.
It may be difficult to distinguish a saccular aneurysm (see Figs. 24.8 and 24.9 ) from an eccentric fusiform aneurysm ( Fig. 24.10A and B ). Eccentric aneurysms are classified as fusiform as long as all three layers of the abdominal aorta are intact. The borders of the eccentric fusiform aneurysm blend into the aortic wall, whereas there is a defect or neck where the saccular aneurysm arises from the aortic wall (see Fig. 24.9 ). Saccular aneurysms tend not to have thrombus deposition (see Fig. 24.9 ) because of their very rapid onset, whereas fusiform eccentric aneurysms evolve more slowly and are therefore more likely to have internal thrombus (see Fig. 24.10 ).
- •
The majority of abdominal aneurysms encountered during a sonographic examination will be fusiform.
- •
If a saccular aneurysm is seen or suspected, the patient should be referred to a surgeon as soon as possible.
- •
It can be difficult to distinguish a saccular aneurysm from an eccentric fusiform aneurysm. The borders of the eccentric fusiform aneurysm blend into the aortic wall, whereas there is a defect or neck where the saccular aneurysm arises from the aortic wall.
Aneurysm formation
The diameter thresholds for abdominal aortic intervention are 5.5 cm in men and 5.0 cm in women. Lower thresholds, such as 5 cm, are being considered for endovascular stent graft placement, but are not yet established in current guidelines. The application of diagnostic ultrasound is based on detecting aneurysms below this threshold to avoid abdominal aortic aneurysm rupture and retroperitoneal hemorrhage. Although patients can be symptomatic (see Table 24.1 ), the use of ultrasound has significantly increased the detection rate for smaller asymptomatic aneurysms.
Rupture of an abdominal aortic aneurysm is a catastrophic event with low survival rates. The risk of rupture is so high for diameters above 5.5 cm ( Fig. 24.11 ) that surgical or endovascular intervention is recommended for almost all aneurysms that approach 5.5 cm. The pathologic processes that lead to the formation of abdominal aortic aneurysm are not fully understood. It is clear that blood pressure, cigarette smoking, and a genetic predisposition are the major factors contributing to aneurysm formation. Aneurysms are also much more common in men than in women, with an estimated ratio of between 4 : 1 and 13 : 1. The commonly held theory that aneurysm formation is an atherosclerotic process needs to be placed in the context of risk factors ( Fig. 24.12A and B ). Elevated blood pressure and cigarette smoking are risk factors that are causally linked to the deposition of lipids and atherosclerosis. In the aorta, these risk factors are associated with the formation of early atherosclerotic plaque. Exposure to cigarette smoke and elevated blood pressures can have deleterious effects on the constituents of the aortic wall. For example, cigarette smoke is associated with increased activity of elastase. Elastase will enzymatically break down the elastin in the aortic wall and compromise mechanical and structural integrity. Persistently elevated blood pressure, especially pulse pressure, produces repetitive mechanical stress on the aortic wall. The pulsatile wall strain and fragmentation of elastin fibers can lead to mechanical fatigue of the aortic wall and diameter enlargement.


In addition, studies show an overall increase in the amount of collagen within the aortic wall with aging (types I and III collagen). This is associated with an overall loss of normal collagen architecture in the media and adventitia, resulting in disordered microstructure in the aortic wall. In combination, these changes in the content of elastin and collagen compromise the elastic integrity of the aorta, weaken the aortic wall, and lead to aneurysm formation.
There are also genetic factors associated with aneurysm formation. The most common is simple inheritance of a familial, mostly male-linked, risk for developing aneurysms. Another type of genetic inheritance includes disease states where the aortic wall is abnormal because of an inherited defect in the structure of fibrillin (gene locus FBN1) such as Marfan and Ehlers-Danlos syndromes.
- •
Major risk factors for aneurysm formation are smoking and elevated blood pressure.
- •
Genetic predisposition for aneurysm formation has familial inheritance and an association with the FBN1 gene locus.
Ultrasound Examination
Examination and measurement
Failed abdominal aortic examinations are often caused by the presence of bowel gas. Fasting after midnight and avoiding actions that lead to air swallowing such as smoking or chewing gum before the examination can decrease bowel gas. In some instances, a bowel preparation can be used to improve image quality.
The examination will typically include the full length of the abdominal aorta from the diaphragm to the iliac artery bifurcation. The common iliac artery bifurcation should be evaluated for extension of an abdominal aortic aneurysm into the iliac artery, common iliac artery aneurysms, and iliac artery stenoses.
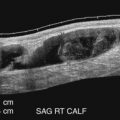
A curved array transducer with a gray-scale frequency of 3 to 5 MHz or greater is normally used. A sector transducer or a linear array transducer can be used, depending on body habitus. We prefer to use one to two focal zones to keep a high frame rate during the examination. Acquired images include transverse views of the proximal, mid, and distal abdominal aorta with associated diameter measurements. Typically, anteroposterior and transverse diameter measurements are made. Anteroposterior measurements are more reliable because they are based on the reflection of the ultrasound beam from the aortic wall interfaces. The best images are obtained when the ultrasound beam is perpendicular to the wall. We also obtain longitudinal (sagittal) images to confirm the anteroposterior measurements ( Fig. 24.13 ). The sagittal images also offer the ability to detect the presence of early aortic ectasia, a focal enlargement of the aortic diameter not yet reaching 3.0 cm ( Fig. 24.14 ).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree