16 Ultrasound Evaluation Before and after Hemodialysis Access
Ultrasound can be extremely useful in the evaluation of the many problems facing the hemodialysis patient. It is a noninvasive technique that can show more vascular detail than physical examination, without the risk for phlebitis or contrast reaction from conventional venography. Current Dialysis Outcome Quality Initiative (DOQI) guidelines encourage the placement of arteriovenous fistulas (AVFs) rather than grafts, because of their greater longevity and decreased incidence of infection.1,2 Detailed evaluation of arterial and venous anatomy before hemodialysis access improves the visualization of veins that may be suitable for native AVF placement, particularly in the patient with a history of prior failed access and/or prior placement of central catheters.
Vascular mapping before hemodialysis access also may change surgical management since the identification of a suitable efferent vein would favor the placement of an AVF rather than a graft. In addition, there is an increased likelihood of selecting the most functional vessels preoperatively, with a subsequent decrease in unsuccessful surgical explorations.3 Vascular mapping has doubled the proportion of patients in dialysis with a fistula in our patient population.4
Despite preoperative vascular mapping, up to 60% of AVFs still fail to mature adequately to support hemodialysis.5 In these patients, ultrasound can determine the potential cause of an immature AVF. It can also determine whether the AVF should be treated by the angiographer (angioplasty of a stenosis) or surgeon (AVF revision or accessory vein ligation).6 Ultrasound is also useful in the evaluation of the patient with a hemodialysis graft, to assess for the presence of a stenosis and determine the etiology of perigraft pulsatile masses (hematoma versus pseudoaneurysm).
Basic Concepts of Hemodialysis Access
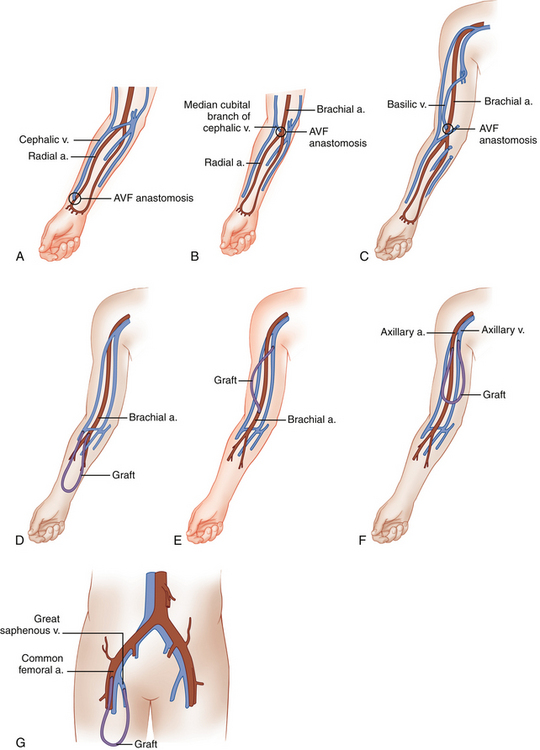
During hemodialysis, two 15-gauge needles are placed into the mature AVF or graft, with the more distal needle (pointing toward the artery) carrying blood from the patient to the dialyzer. The second needle is placed more proximally (closer to the venous outflow) in the AVF or graft and returns the blood to the patient’s circulation. Artery/vein configurations for the various types of AVFs and grafts are shown in Table 16-1. Figure 16-1 shows anatomic drawings of the three most common AVFs and four most common graft configurations.
TABLE 16-1 AVF and Graft Artery/Vein Anastomoses
| AVF Types | Artery | Vein |
|---|---|---|
| Forearm cephalic vein | Radial | Cephalic |
| Forearm vein transposition | Radial | Ulnar, dorsal, or volar vein |
| Upper arm cephalic vein | Brachial | Cephalic |
| Basilic vein transposition | Brachial | Basilic |
| Graft Types | Artery | Vein |
| Forearm loop | Brachial | Antecubital |
| Upper arm straight | Brachial | Basilic |
| Upper arm loop | Axillary | Axillary |
| Thigh graft | Common femoral/superficial femoral | Great saphenous/common femoral |
AVF, arteriovenous fistula.
Surgeons prefer to create a hemodialysis access in the nondominant arm, because the activities of daily living can be carried out with the dominant arm while the nondominant arm is healing after surgical access placement. However, most will put an AVF in the dominant arm before a graft is considered. An AVF is placed first in the forearm, if the patient has suitable anatomy, and the upper arm is then “saved” for future potential dialysis access. Likewise, if an AVF cannot be placed, a forearm graft is preferred to placement of an upper arm graft. If no upper extremity graft options are available, a thigh graft is preferable to dialyzing with an indwelling venous catheter.7 A list of the preferred order of access placement is given in Table 16-2. In general, an AVF or graft will provide higher dialysis flow rates than a tunneled catheter,8 with a lower infection rate.9
TABLE 16-2 Preferred Order of Access Placement
| Order of Access Placement Type |
| 1. Nondominant forearm cephalic vein fistula |
| 2. Dominant forearm cephalic vein fistula |
| 3. Nondominant or dominant upper arm cephalic vein fistula |
| 4. Nondominant or dominant upper arm basilic vein transposition fistula |
| 5. Forearm loop graft |
| 6. Upper arm straight graft |
| 7. Upper arm loop graft (axillary artery to axillary vein) |
From Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions, Kidney Int 62:1109–1124, 2002.
Vascular Mapping Before Hemodialysis Access Placement
General Principles
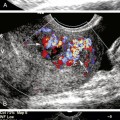
The protocol for vascular mapping before hemodialysis access placement has been previously described.3,10 A high-resolution linear ultrasound transducer is used to evaluate the arm vessels (generally 9 MHz or higher). Gray-scale transverse plane imaging is used to identify vessels (artery and vein) and evaluate their diameter and wall thickness. Sequential vein compression is used to assess for compressibility, indicating patency, or loss of compressibility due to acute or chronic thrombus. The depth from the skin surface of the anterior wall of the cephalic vein is measured. Color Doppler images and Doppler spectral waveforms are obtained in the longitudinal plane of vessels selected for potential vascular access.
The forearm veins and arteries are assessed to determine whether the patient is a candidate for a forearm AVF, the most desirable initial type of hemodialysis access. If vascular anatomy suitable for forearm fistula creation is not found, the upper arm vessels should be mapped. Arteries should be assessed for intimal thickening, calcification, and stenosis. The presence of significant concentric calcification should be noted, because the artery may be too calcified for successful surgical access creation. An important aspect of planning for AVF and graft creation is vessel size assessment. Minimum artery and vein diameters are shown in Table 16-3. It is important to note that upper extremity vessel mapping should be performed with the patient sitting upright, so the arm veins are dependent to the heart. This position will optimize vein diameter, along with tourniquet use. Ambient room temperature should not be cold, and warm blankets or warm compresses can be used as necessary.
TABLE 16-3 Minimum Diameter Criteria for AVF and Graft Creation
| Vessel | Minimum Diameter (cm) |
| AVF vein | 0.25 |
| Graft vein | 0.40 |
| Artery (graft or AVF) | 0.20 |
AVF, arteriovenous fistula.
From Silva MB, Hobson RW, Pappas PJ, et al: A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation, J Vasc Surg 27(2):307–308, 1998.
Forearm Assessment
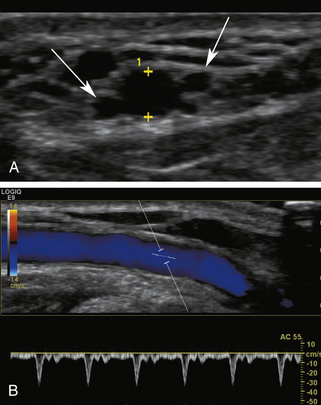
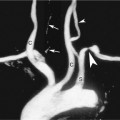
The nondominant forearm is assessed first. The patient’s arm is placed in a comfortable position on towels on top of a procedure stand (Figure 16-2) or phlebotomy chair. The internal diameter of the radial artery at the wrist should measure at least 2 mm (Figure 16-3). If the radial artery internal diameter is not satisfactory within several centimeters of the wrist, the internal diameter of the ulnar artery at the wrist is measured. If the diameter of the ulnar or radial artery is not at least 2 mm in the lower third of the forearm, the forearm radial and ulnar arteries are assessed on the dominant side. If no forearm artery is satisfactory, the patient is not a candidate for a forearm AVF.
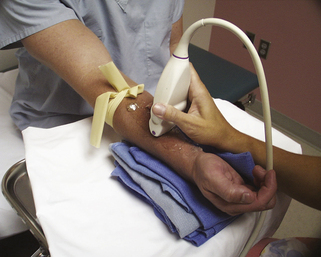
FIGURE 16-2 Proper position of arm on instrument stand for imaging of the vessels for hemodialysis planning.
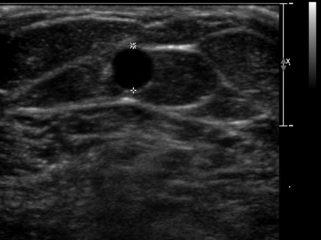
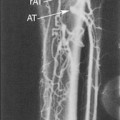
If the radial or ulnar artery at the wrist meets size criteria, the next vessel to evaluate is the cephalic vein. A tourniquet is placed moderately tightly at the midforearm. The entire distal forearm is percussed, similar to starting an intravenous line, for approximately 3 minutes. All wrist veins that measure greater than 2 mm after the 3-minute tapping are evaluated. Veins smaller than 2 mm will probably not dilate up to an adequate size. However, veins greater than 2 mm may dilate to greater than 2.5 mm with more focused tapping and/or a warm compress. Special attention is given to the cephalic vein, as this is the preferred venous outflow conduit. The cephalic vein area is assessed for the presence of a continuous vein up to the tourniquet with a diameter of at least 2.5 mm (Figure 16-4).
If the forearm portion of the cephalic vein is adequate, a tourniquet is placed at the elbow and the vein is followed in a cephalad direction to the elbow. If the vein is discontinuous or stenotic, it is not satisfactory for fistula creation. Then, a search for another adequate forearm vein should be performed. Branch points should be assessed carefully, because vein narrowing below 2.5 mm may occur at branch points. Following examination of the forearm, the tourniquet is moved to the axilla, and the vein of interest is followed centrally to ensure that it empties into the deep venous system. The tourniquet must be removed to evaluate the cephalic vein insertion into the subclavian vein. Occasionally, overlying muscle may give the appearance of a stenosis at the insertion of the cephalic vein into the subclavian vein. Placing the patient’s arm by his/her side (rather than in an abducted position) may alleviate an apparent cephalic vein stenosis.