Ectopic pregnancy refers to any pregnancy that occurs outside of the uterine cavity. It is reported to occur in approximately 2% of all pregnancies in the United States. Its incidence has increased substantially during the past 25 years, as a result of increasing frequency of pelvic inflammatory disease and use of infertility treatments. In pregnancies achieved via in vitro fertilization (IVF), its incidence has been estimated at 2.2% to 5.6%. The classic clinical triad of ectopic pregnancy is amenorrhea, vaginal bleeding, and abdominal pain, but this symptom complex occurs in fewer than 50% of patients who present with ectopic pregnancy.
The main diagnostic tests for ectopic pregnancy are serum β-human chorionic gonadotropin (β-hCG) and ultrasound (US). The diagnosis should be considered in a woman who has a β-hCG over the “discriminatory level”—the level above which an intrauterine pregnancy is consistently visible on US—if no gestational sac is identifiable on her sonogram. The diagnosis is especially likely if US demonstrates an adnexal extraovarian abnormality. Ectopic pregnancy is established with certainty when US shows a definite gestational sac (a fluid collection containing an embryo with heartbeat or a yolk sac) outside of the uterine cavity.
More than 90% of ectopic pregnancies are tubal, occurring in the extracornual portion of the fallopian tube, most commonly within the ampullary segment. Other types of ectopic pregnancy include those implanting in the cervix, the interstitial segment of a fallopian tube (the portion passing through the cornu), or a cesarean section scar, and ectopic pregnancies that occur concomitantly with an intrauterine pregnancy (heterotopic pregnancy). These unusual ectopic pregnancies, which are uncommon in pregnancies conceived naturally but more frequent in those achieved via IVF and other assisted reproductive technologies, are associated with greater morbidity than tubal ectopic pregnancies.
Treatment options for tubal ectopic pregnancy are well established and have been proven effective. These include systemic methotrexate injection (injected intramuscularly via a single- or multiple-dose regimen) and surgery (open or laparoscopic). The decision between these is based on a number of factors, including size of the adnexal mass, β-hCG level, and presence or absence of an embryonic heartbeat.
These treatments, however, are less well suited to unusual ectopic pregnancies. Systemic methotrexate has been found to be far less effective for uncommon ectopic pregnancies than for most tubal ectopic pregnancies because pregnancies implanted in the cervix, interstitial segment of the fallopian tube, or cesarean section scar will often continue to grow after systemic methotrexate injection. Surgery is also less well suited to unusual ectopic pregnancies because more involved major surgery would be required for cervical, interstitial, or cesarean scar pregnancies than for tubal ectopic pregnancies. In view of these limitations of systemic methotrexate and surgery, minimally invasive US-guided treatment options for these unusual ectopic pregnancies have been developed, and have proven to be safe and effective.
Cervical Ectopic Pregnancy
Cervical ectopic pregnancy (also termed cervical pregnancy ) occurs when the fertilized ovum implants within the endocervical canal. Cervical pregnancies are uncommon, occurring in at most 1 of 2500 pregnancies and less than 0.5% of all ectopic pregnancies. Cervical pregnancies are much more common in pregnancies achieved via IVF, where they have been estimated to have an incidence of 0.1% of all pregnancies and 3.7% of ectopic pregnancies. Risk factors, aside from IVF, include Asherman’s syndrome, use of an intrauterine device, and previous history of cesarean section delivery, dilatation and curettage (D&C), or pelvic inflammatory disease. Because the cervix is composed mainly of fibrous connective tissue and only 15% to 20% smooth muscle, it provides an unfavorable environment for decidual response and placentation. Women with cervical ectopic pregnancies often experience severe bleeding, which explains the high maternal morbidity and mortality associated with this entity.
Before the advent of US, the diagnosis of cervical pregnancy was often made at pathology after hysterectomy for uncontrollable vaginal bleeding. US now permits the diagnosis to be made noninvasively and earlier in gestation, which has opened the door to conservative, uterus-sparing, treatment options.
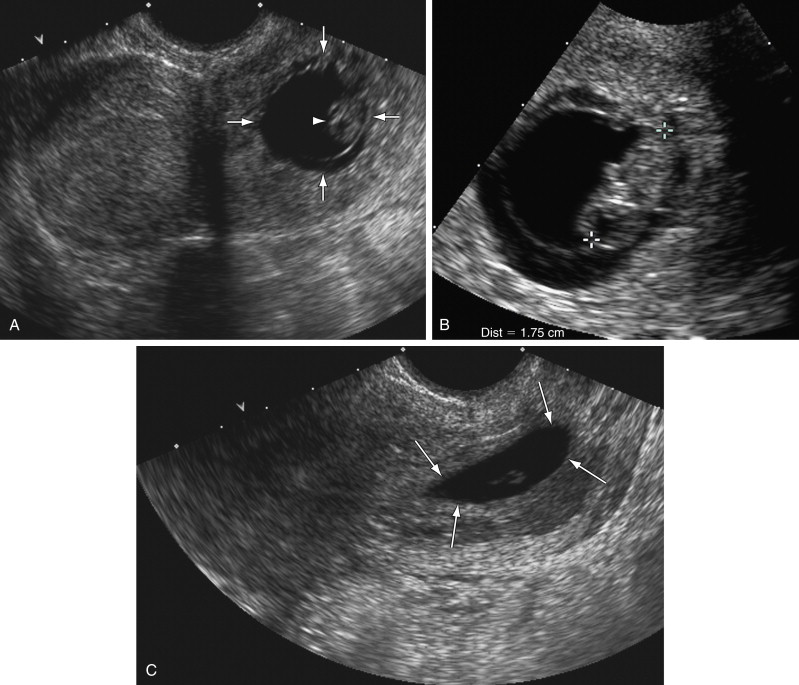
The initial report of sonographic diagnosis of cervical pregnancy, in 1978, concluded that the diagnosis could be suggested when US demonstrated an enlarged cervix, amorphous intrauterine echoes, and absence of intrauterine pregnancy. With increased resolution of US and the introduction of transvaginal sonography, the diagnosis can be made more accurately and as early as 5 to 6 weeks of gestation. The diagnosis is made when a gestational sac is seen within the cervix and the alternative explanation for an intracervical sac—a spontaneous abortion in progress—is excluded. Findings indicative of a cervical pregnancy are a well-formed round or oval intracervical sac with prominent surrounding echogenic rim (representing trophoblastic tissue), whereas a flattened or crenated sac with thin surrounding rim is characteristic of a spontaneous abortion in progress ( Figure 41-1 ). An embryo with cardiac activity, if present, provides further support for a cervical implantation as opposed to a sac passing through the cervix. Pressure with the transvaginal probe can also help to arrive at a correct diagnosis: If the sac slides relative to the cervical canal, it is likely to be a spontaneous abortion in progress, whereas lack of sliding suggests a cervical implantation. If the distinction between cervical pregnancy and spontaneous abortion in progress cannot be made with certainty and the patient is hemodynamically stable, a follow-up scan 1 day later can resolve the uncertainty because a spontaneous abortion in progress will likely have passed.
Since the 1980s, a variety of conservative approaches to treatment of cervical pregnancies have been used. These include uterine artery embolization and cervical curettage, single- or multiple-dose systemic methotrexate, and US-guided local injection of methotrexate or potassium chloride (KCl) into the gestational sac. These treatments are sometimes used in combination, either by design (e.g., some authors advocate using systemic methotrexate in conjunction with local injection of methotrexate or KCl) or when needed in specific cases (e.g., using uterine artery embolization to stop otherwise uncontrollable bleeding after local injection). Overall, these conservative treatments can successfully avoid the need for hysterectomy in more than 95% of patients with cervical pregnancies (although cervical curettage alone has a high rate of hemorrhage requiring hysterectomy).
The least invasive treatment is systemic methotrexate, either single or multiple doses, injected intramuscularly. Some authors, however, have found this form of treatment to have a substantial failure rate. In one series, systemic methotrexate alone failed in 6 of 11 (54.5%) cervical pregnancies when a heartbeat was present, and in 2 of 11 (18.2%) when there was no heartbeat. Features, other than presence of a heartbeat, that are associated with a high failure rate include a gestational age of 9 weeks or greater and β-hCG of greater than 10,000 mIU/mL. Systemic methotrexate may also cause side effects, including stomatitis and transient liver enzyme elevation.
The first reported case of US-guided minimally invasive treatment of cervical pregnancy, using local intrasac injection of methotrexate, was published in 1989. Since then, at least 77 cases of local injection, using either methotrexate or KCl, have been reported, and many more have undoubtedly been performed. The procedure does not require hospital admission and is best performed no later than 8 to 9 weeks of gestation. No preprocedural sedation or prophylactic antibiotics are necessary, but administration of oral analgesics may be helpful. The procedure begins with a povidone–iodine prep of the cervix. A sterile cover is placed on the transvaginal probe, and a needle guide is secured. Sterile gel is applied to the end of the probe, which is then inserted into the vagina. A 20-gauge needle, 20 to 25 cm in length, is inserted through the needle guide and advanced into the gestational sac. Once the needle tip is in place, 2 to 5 mL of KCl (at a concentration of 2 mEq/mL) or 25 to 50 mg of methotrexate is instilled under continuous US guidance ( Figure 41-2 ), and the needle is removed. (Note: If KCl is used and there is an embryonic heartbeat before the procedure, then the procedure is not complete until the cardiac activity has ceased.) After the procedure, it generally takes 1 to 3 months for complete resolution: return to a normal sonographic appearance and conversion to a negative β-hCG.

Based on the published results, two main treatment options for cervical pregnancy should be considered:
Option 1: US-guided local injection of methotrexate or KCl into the gestational sac (or embryo)
Option 2: A trial of systemic methotrexate (single or multiple dose), followed by local intrasac injection if the systemic treatment fails
Option 1 is optimal for patients who want immediate definitive treatment, whereas option 2 is best for patients who prefer to avoid the more difficult intrasac injection. Uterine artery embolization may be needed for control of bleeding in a small fraction of patients after either option 1 or 2, but there is no reason or need to use it routinely.
Interstitial Ectopic Pregnancy
The terms interstitial pregnancy and cornual pregnancy are sometimes used synonymously, referring to a pregnancy implanted in the interstitial portion of the fallopian tube (the part of the tube that travels through the cornu of the uterus). Some authors, however, believe that interstitial pregnancy is the most appropriate term and consider a cornual pregnancy to be different (see Chapter 22 ). Interstitial pregnancies constitute 2% to 3% of all ectopic pregnancies. They are somewhat more frequent in IVF pregnancies, where they make up approximately 7% of all ectopic pregnancies.
The pathogenesis is unknown but is thought to relate to an abnormal transport of the fertilized ovum within the fallopian tube. Risk factors, other than IVF, include uterine anomalies, previous ectopic pregnancy, pelvic inflammatory disease, and presence of an intrauterine device. Interstitial pregnancies carry a maternal mortality rate of approximately 2%, considerably higher than that of other tubal ectopic pregnancies. The higher mortality rate may be related to the location of the implantation site between the uterine and ovarian arteries, which can result in massive internal hemorrhage. Another contributing factor in some cases is a delay in diagnosis because of failure to distinguish between normal intrauterine versus interstitial implantation.
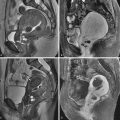
Diagnosis of interstitial pregnancy is generally made by US. Sonographic findings ( Figure 41-3 ) include eccentric (superolateral) location of the gestational sac within the uterus, with little or no myometrium around the lateral aspect of the sac. The presence of an “interstitial line sign”—an echogenic line extending from the uterine cavity to the edge of the gestational sac—may be helpful in the diagnosis. Other sonographic techniques that can be useful include real-time observation while moving the transvaginal probe, as well as three-dimensional sonography. The former can help distinguish an interstitial pregnancy from other tubal pregnancy, in that the uterus and gestational sac will move in unison if the sac is in the interstitial portion of the fallopian tube, whereas they may move separately if the gestational sac is located more distally in the fallopian tube. Three-dimensional sonography can aid in the diagnosis by clearly depicting the uterine anatomy, including the relationship between the uterine body and cornua.