Introduction
Vascular emergencies require a prompt diagnosis, as timely intervention is often critically important. Delays of minutes or hours in diagnosis may mean the difference between life and death, or limb preservation or loss. Ultrasound plays an important role in the diagnosis and management of many vascular emergencies.
Because vascular emergencies are often managed nonoperatively, an accurate diagnosis is needed for appropriate patient triage. Several diagnostic modalities can be applied in such cases. In addition to duplex ultrasound, other modalities include computed tomographic angiography (CTA), magnetic resonance angiography (MRA), digital subtraction angiography, and intravascular ultrasound. The advantages of ultrasound for emergencies include availability, portability, speed, and high temporal and spatial resolution. A relative disadvantage is the acoustic barrier presented by bone, air, or tissue edema; these limit the use of ultrasound at the skull base, in the chest, deep pelvis, and injured extremities with extensive tissue disruption. Regional arterial supply through collateral pathways around a diseased vessel is less comprehensive with ultrasound than with angiography. The choice of ultrasound as a diagnostic modality reflects these factors and the clinical information needed to direct management.
Ruptured Abdominal Aortic Aneurysm
An aneurysm is a localized dilation of an artery, with an increase in diameter of greater than 50% of the normal size. Abdominal aortic aneurysms (AAAs) are most commonly encountered in the infrarenal region. In general, AAAs occur when the maximal transverse diameter is greater than 3 cm. As the population ages, the incidence of AAAs is increasing. Approximately 1.5 million Americans have AAAs, and 200,000 are diagnosed each year. Most AAAs are asymptomatic and are detected during routine physical examinations or radiologic procedures for other problems. Elective repair is reserved for subjects with AAAs of at least 5 cm in diameter, and postoperative survival is approximately 95%. Symptomatic aneurysms may result in abdominal, flank, or back pain. Complications of aortic aneurysm include distal embolization, vascular thrombosis and rupture of the aneurysm. The latter complication is usually fatal if untreated; surgical repair carries a 30% to 65% survival rate. Success is highly dependent on rapid diagnosis and transport to the operating room.
Ultrasonography is often used as the initial procedure for diagnosis of AAAs. It is also used for screening and serial measurement of AAA size. Rapid enlargement of AAA on serial scans is worrisome for impending rupture. When aneurysms rupture, only 50% of patients present with the “classic” triad of abdominal or back pain, hypotension, and pulsatile abdominal mass. The clinical diagnosis may therefore pose a challenge, particularly in patients without a known history of AAA. An accurate imaging diagnosis of retroperitoneal hematoma in the presence of AAA enables prompt initiation of endovascular or surgical treatment.
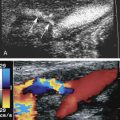
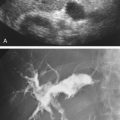
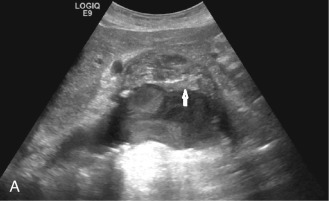
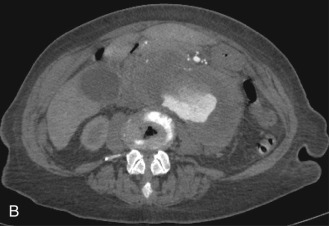
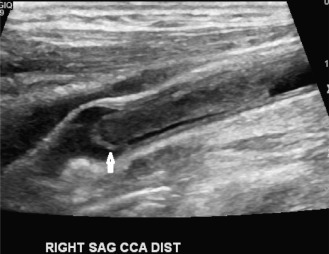
Although computed tomography (CT) is useful for this purpose, ultrasound is more expeditious in these potentially unstable patients. Ultrasound diagnosis of an abdominal aneurysm has an accuracy of 98%. A combination of sonographic confirmation of aneurysm in the setting of abdominal pain and hemodynamic instability resulted in the correct decision to intervene in 95% of patients. Although active extravasation is usually not brisk enough to demonstrate by Doppler ultrasound, the resultant hematoma provides evidence of rupture ( Fig. 17.1 ). Initially, intramural hemorrhage may be visible as an echogenic crescent within the aneurysmal wall. Following rupture, blood initially accumulates in the para-aortic space, extending toward the flanks via the pararenal space. Hemorrhage may track along the course of the iliac arteries to the extraperitoneal spaces of the pelvis ( Fig. 17.2 ). Anterior extension may transgress the posterior peritoneum, resulting in hemoperitoneum. Additional findings include AAA deformation, luminal thrombus inhomogeneity, and interruption of aneurysmal wall or thrombus.
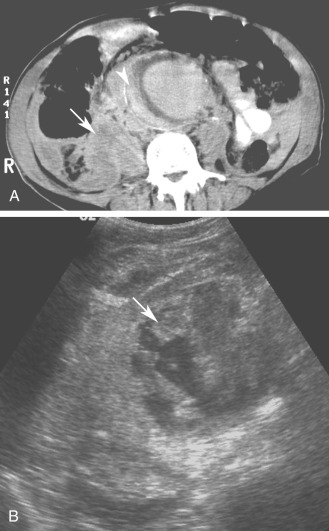
When the patient presents with abdominal pain, the operator may be reluctant to use probe pressure to visualize the aorta for fear of provoking or exacerbating rupture. Although this is rarely a practical concern, a coronal approach from the left flank avoids trapping the aneurysm between the transducer and the spine. Additionally, this approach frequently provides improved visualization of the aorta by circumventing overlying bowel gas.
Nonetheless, conventional sonography has some limitations in imaging patients with a ruptured aneurysm because retroperitoneal hematoma is not always readily apparent, and the rupture itself is seldom directly visualized. In the appropriate clinical setting, contrast-enhanced sonography can be performed in less time than is required for a contrast-enhanced CT. Findings associated with aneurysm rupture in these studies include delayed or protracted aortic lumen opacification, contrast leakage through luminal thrombus or around the aneurysm, and dependent contrast pooling around the aneurysm.
Aortic dissection may be encountered as an alternative diagnosis in patients with a clinical presentation suggesting aneurysm rupture. Although ultrasound is not the primary diagnostic modality because it cannot reveal the full extent of dissection in the thorax, the characteristic intimal flap and altered blood flow pattern are readily visible on abdominal sonography and can lead to appropriate workup and treatment ( Fig. 17.3 ). In one study, the use of contrast-enhanced sonography increased the sensitivity for abdominal aortic dissection to 97% from 68% for combined gray-scale and duplex sonography. Additional details about aortic aneurysm and dissection are presented in Chapter 24 .
- •
AAAs are commonly infrarenal and occur when the maximal diameter is greater than 3 cm.
- •
Complications of aortic aneurysm include distal embolization, vascular thrombosis, and rupture of the aneurysm.
- •
Findings of aneurysm rupture include intramural hemorrhage, para-aortic hematoma, hemoperitoneum, AAA deformation, luminal thrombus inhomogeneity, and interruption of aneurysmal wall or thrombus.
- •
Aortic dissection is an alternate diagnosis in patients suspected of AAA rupture. The characteristic intimal flap and altered blood flow pattern are readily visible on abdominal sonography and can lead to appropriate workup and treatment.
Carotid Artery Stenosis/Thrombosis
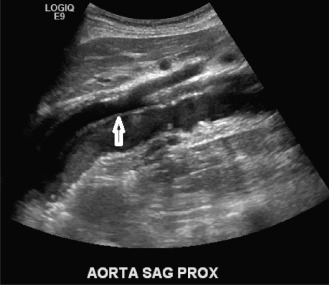
Symptomatic carotid artery stenosis is manifested by transient ischemic attacks (TIAs) or stroke (cerebrovascular accident, CVA). Neurologically unstable patients in whom there is no evidence of intracranial hemorrhage are potential candidates for emergency carotid endarterectomy. These cases include patients presenting with crescendo TIAs, stroke in evolution, fluctuating or fixed neurologic deficits caused by acute carotid artery thrombosis, and free-floating thrombus. In these cases, rapid sonographic evaluation of the carotid bifurcation can result in timely intervention to prevent stroke or death.
Sonographic detection and quantification of carotid stenosis in the emergency setting (i.e., progressive neurologic deficit) is similar to the nonacute setting and is described in Chapter 7 . It is important to distinguish acute carotid thrombosis from stable (chronic) carotid occlusion. Whereas revascularization of a chronic occlusion is generally contraindicated, timely intervention may avoid or limit neurologic sequelae in cases of acute thrombosis.
Acute thrombosis may occur as a complication of endarterectomy or stenting, or it may represent acute progression of carotid stenosis. The thrombus is usually heterogeneously echogenic ( Fig. 17.4 ). The vessel lumen is of normal caliber or slightly expanded, as opposed to chronic occlusion, which may result in luminal narrowing or obliteration. Pulsations may be observed in the vessel wall, which retains its normal compliance. Swirling, sludge-like flow may be observed in the carotid bulb at the interface of the thrombosed and patent lumen. Spectral waveforms assume a high-impedance, hammer-like or to-and-fro configuration proximal to the thrombus. Internalization of the external carotid artery (ECA) waveform is uncommonly seen with acute thrombosis because of insufficient time to develop collateral pathways.
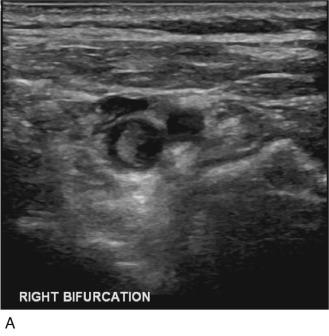
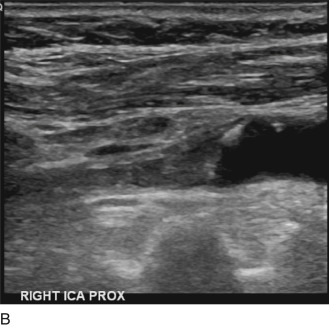
If the thrombus is partially occlusive, its free edge may oscillate back and forth in the bloodstream; the “free-floating” thrombus has a characteristic appearance at real-time examination ( Fig. 17.5 ). The risk for embolization is related to the extent of attachment of the base of the thrombus to the vessel wall; this can be depicted with the aid of color Doppler or B-flow imaging.
When patients with acute carotid thrombosis undergo thrombectomy or revascularization, intraoperative ultrasound is a useful adjunctive technique. Scanning directly over the vessel in the exposed surgical field yields superb resolution of the vessel wall. It is possible to visualize small intimal flaps, ulcerative plaques, or retained thrombi, leading to immediate surgical revision, which favorably impacts patency rates.
- •
Acute carotid thrombosis may occur as a complication of endarterectomy or stenting, or it may represent acute progression of carotid stenosis.
- •
The thrombosed carotid lumen is of normal caliber or slightly expanded, as opposed to chronic occlusion, and may be filled with hypoechoic or heterogeneous thrombus.
- •
Spectral waveforms assume a high-impedance, hammer-like or to-and-fro configuration proximal to the thrombus.
Carotid Artery Dissection
There are two types of carotid artery dissection. The “primary” dissection is in the cervical internal carotid resulting from hemorrhage into the wall of the carotid artery and extending distally to the carotid canal in the petrous portion of the temporal bone. The secondary type of carotid dissection usually extends into the common carotid artery (CCA) from a type A dissection of the thoracic aorta.
We will be discussing the primary type of internal carotid artery (ICA) dissection where the media is the most common location for hemorrhage, with extension into the subintimal or subadventitial layers. The former may result in thrombosis of the vessel; aneurysms may occur from the latter. ICA dissections can be spontaneous or associated with trauma. Spontaneous dissections may be associated with type IV Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, and cystic medial necrosis; however, most spontaneous dissections are idiopathic. They often happen in previously healthy individuals younger than 40 years. Most traumatic carotid dissections result from motor vehicle accidents in which the neck is hyperextended, compressing the carotid artery against the atlas or second cervical vertebra. Blunt trauma, penetrating injuries, and catheter injuries during arteriography also cause traumatic dissections.
In the United States, the annual incidence of symptomatic carotid artery dissection is 2.6 per 100,000. The actual incidence of carotid dissection may be higher because many episodes may be asymptomatic or cause only minor transient symptoms, thereby remaining undiagnosed. Morbidity from carotid artery dissection varies in severity from transient neurologic deficit to permanent deficit and death. Dissection of the intracranial portion of the ICA, although rare, is associated with a 75% mortality rate. The male-to-female ratio for carotid dissection is 1.5 : 1. The mean age for extracranial ICA dissection is 40 years; intracranial dissections are more common in patients 20 to 30 years old. Approximately 20% of strokes in young patients are caused by carotid artery and vertebral artery dissections in the neck, compared with 2.5% in older patients.
Patients with carotid dissection often complain of neck pain, headache, tinnitus, or a focal neurologic deficit. Horner syndrome, transient monocular blindness, neck swelling, and cranial nerve palsy may also result. The onset of symptoms may be hours to days after the dissection occurs.
Duplex scanning of patients with suspected carotid dissection can be performed quickly in the emergency setting to make this diagnosis. Findings with duplex scanning include a patent carotid bifurcation with tapering of the ICA, leading to a distal stenosis or occlusion. Spectral analysis can reveal high-resistance waveforms with reduced flow velocity in the ICA ( Fig. 17.6 ). Intimal flaps or membranes may be seen with two patent channels or a thrombosed false lumen ( Fig. 17.7 ). The sensitivity of color Doppler ultrasound is 95% to 96% for diagnosis of internal carotid dissection causing carotid territory ischemia and 71% for dissection causing no ischemic events. Color may obscure the intimal flap, and care must be taken to check the vessel with gray-scale imaging. Sensitivity for extracranial vertebral artery dissection is 75% to 86%. Follow-up scanning can be useful to monitor vessel recanalization and to determine the duration of anticoagulation therapy.
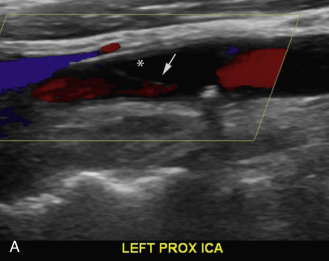
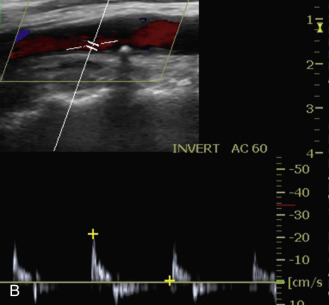
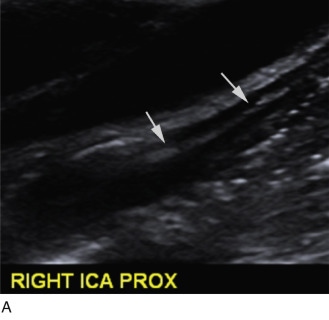
The false lumen may be thrombosed or patent. If acutely thrombosed, the intimal flap may bulge in convex fashion toward the true lumen (see Fig. 17.6A ). A patent false lumen usually exhibits flow characteristics differing from the true lumen, unless a second intimal tear downstream reestablishes continuity between the true and false lumen. More commonly, the false lumen demonstrates low peak velocity with reversal of flow during diastole (to-and-fro pattern; see Fig. 17.7B ).
If the site of dissection is near the skull base, it may not be visible with ultrasound. In such cases, altered hemodynamics in the ICA proximal to the dissection (reduced peak velocity, increased impedance) constitute indirect evidence for this diagnosis in a patient with a compatible clinical presentation. Magnetic resonance imaging (MRI) will typically show the presence of acute thrombus as a zone of increased T1 signal in the wall of the ICA. MR or CT angiography (MRA, CTA) can confirm the presence of luminal narrowing and establish the extent of dissection.
- •
Carotid artery dissection may be primary, involving the cervical ICA and extending distally, or secondary, from extension of type A thoracic aortic dissection.
- •
Most spontaneous dissections are idiopathic, but can also be caused by type IV Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, and cystic medial necrosis.
- •
Traumatic dissections are usually related to motor vehicle accidents.
- •
Typical findings of carotid dissection include a patent carotid bifurcation with tapering of the ICA, leading to a distal stenosis or occlusion. Intimal flaps or membranes may be seen, with two patent channels or a thrombosed false lumen.
- •
High-resistance, low-velocity carotid blood flow suggests distal obstruction such as distal ICA thrombosis or dissection.
Acute Lower Extremity Ischemia
Acute ischemia of the lower extremity is caused by an embolism from the heart or a proximal arterial location, or from acute thrombosis of the affected artery. Approximately 80% of peripheral arterial emboli originate in the heart, often secondary to myocardial infarction, endocarditis, or arrhythmia. An embolism can also originate from any artery outside the heart, and the abdominal aorta is the most common source of artery-to-artery emboli. Atherosclerotic plaques and small aneurysms of the aorta or iliac arteries account for approximately 70% of these emboli. More distal lower extremity arteries (e.g., popliteal) account for most of the other cases of emboli of arterial origin. Duplex imaging of infrainguinal occlusive disease has become popular in elective revascularization cases, leading some practitioners to abandon angiography. The same arguments supporting preferential use of ultrasonography in acute situations will undoubtedly lead to its use in cases of acute arterial thrombosis.
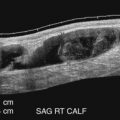
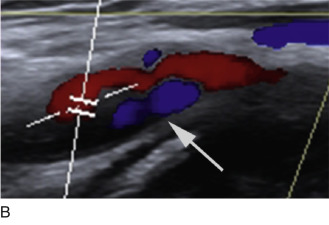
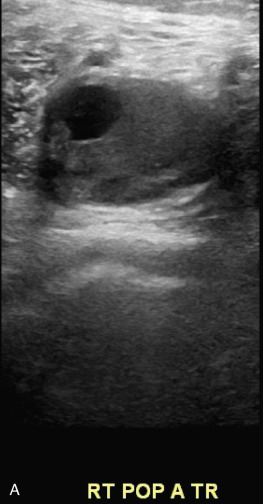
Detection of lower extremity aneurysms that may be the source of emboli is readily accomplished with ultrasound. The upper limit of the normal arterial diameter is 10 mm in the common femoral artery, 8 mm in the superficial femoral artery, and 5 to 6 mm in the popliteal artery. Fusiform enlargement is often accompanied by vessel tortuosity and may be multifocal. Popliteal aneurysms are associated with AAA in 30% to 35% of cases. Intraluminal plaque and thrombus with an irregular luminal contour and heterogeneous echogenicity may imply higher risk for embolization than smooth, homogeneous plaque without thrombus ( Fig. 17.8 ). Acute limb-threatening ischemia may be the initial presentation of popliteal aneurysms; although the diagnosis may be missed by contrast angiography, the aneurysm is readily detected with duplex sonography.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree