Fig. 4.1
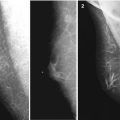
Breast carcinoma in a man. (a) Photo. (b) Scheme
Despite characteristic features, only one third of men with breast cancer visit their doctor within 1 month after detection of the disease. The other two thirds of men with breast lesions do not seek medical care for months and sometimes years. According to V. P. Letyagin (2006), the primary reason for consulting a doctor in 21 % of cases was long-lasting breast skin ulceration. However, late stages of breast carcinoma at diagnosis in men are not only caused by men not seeking medical consultation promptly. Sometimes, it is a consequence of inefficient primary medical care because of low oncologic awareness. Breast cancer was not suspected in approximately 20 % of men at the first referral to a doctor (Letyagin 2006).
Poor development of subcutaneous fat and affinity to the skin and underlying tissues mean that the tumor becomes immobile very early in relation to the anterior thoracic wall, and the skin over the tumor fixes and dimples. Even superficial palpation permits identification of an irregular lesion of cartilaginous density near the nipple and areola. While examining the status of the nipples and areolas, it is necessary to pay attention to thickening of the skin, retraction or dimpling, and discharge from the nipples. Skin ulceration over the tumor in men appears significantly earlier than in women.
The average size of a diagnosed tumor is usually 2–4 cm and may vary from 0.5 to 12.5 cm. Breast cancer is more often observed in the left breast, with a ratio of 1.07–1.63:1 (Ostrovskaya et al. 1988; Akimov 1992; Contractor et al. 2008). The reason for that is unclear, but a similar observation is noted in women.
The same histological types of breast carcinoma (preinvasive ductal and lobular, infiltrative ductal) are observed in men as are seen in women. Some additional breast malignancies described in men were inflammatory, lobular, papillary, ductal cancer in situ, and atypical hyperplasia of ductal epithelium (Giordano et al. 2004). Some observations of tubular cancer characterized with high differentiation and good prognosis were published (Scheike 1975). Single instances of breast sarcomas in men were described. Their histological types were quite different: fibrosarcomas, leiomyosarcomas, neurosarcomas, reticulosarcomas, lymphosarcomas, and carcinosarcomas. In most cases, histology of the cancer reveals typical ductal cancer with the structures of cribrous, papillary, and solid types, and, rarely, lobular, comedocarcinoma, and adenocarcinoma. Cystic transformation is typical, whereas invasive growth is reported less often (Paltsev and Anichkov 2005).
According to O. V. Akimov (1992), there is no significant difference in the incidence of intraductal, invasive ductal, and other (mucous, medullary, and papillary) carcinoma types. The most frequent finding in men, as well as in women, is invasive ductal cancer (80.2 and 82.0 %, respectively). Intraductal carcinoma in men and women also exhibits practically identical incidence (1.3 and 2.3 %, respectively). Special histological types of cancer were detected in 20.0 % of cases in men and 13.0 % of cases in women. The comparison of the degree of differentiation of invasive ductal breast cancers in men and women revealed that carcinoma in men is usually less differentiated than in women. Highly differentiated carcinoma in men was not reported at all, as compared with 5.9 % in women. In the group of special histological types, tubular adenocarcinoma in men was not observed, whereas its incidence in women was 3.6 %. Clinical signs of Paget disease of the breast are described in detail in women and are practically the same in men. It is an extremely rare condition that accounts for less than 1 % of all male breast cancers. Inflammatory breast carcinoma in men is rare and extremely aggressive. It is likely that not all researchers include this type of cancer in the description of their own observations. N. Treves (1959) specifies that the frequency of inflammatory cancer in men is slightly less than 2 %.
Male breast cancer has a bad prognosis. It develops quickly and metastasizes early. The 5-year survival rate is 20–30 % (Ostrovskaya et al. 1988).
4.2 Ultrasound Signs of Breast Cancer in Men
The first publications devoted to the differential diagnosis of breast pathology with ultrasound (US) technologies in A-mode belong to J. J. Wild and D. Neil (1951) and in B-mode to J. J. Wild and J. M. Reid (1952). Before the mid-1970s, subcentimetric breast lesions could be successfully detected only in 8 % of cases. In the 1980s, echography was considered an additional diagnostic procedure to clinical survey and mammography. US today is a highly effective and obligatory method of examination, which is used in men along with mammography, clinical survey, and palpation in the diagnosis of breast pathology, including early and differential diagnosis of malignancies (Zabolotskaya and Zabolotsky 1997; Trofimova 2000a, b; Korzhenkova 2004; Komarova 2006).
US examination is based on the ability of tissues with different acoustic resistances to reflect US (cyclic sound pressure waves with a frequency greater than 20,000 Hz). Modern US scanners work in real time, which provides an opportunity to observe the locomotion of organs in a natural time course. The development of new diagnostic equipment, the introduction of digital US scanners, the use of modern high-frequency probes of 7.5–18 MHz, and the complex use of modern options and technologies significantly increased the diagnostic possibilities of sonography.
The complex of modern US technologies for the diagnosis of breast cancer confers the following options:
1.
Grayscale regimen
2.
Tissue harmonics
3.
Adaptive coloring
4.
Color Doppler imaging
5.
Power Doppler mapping
6.
Three-dimensional reconstruction of grayscale images, real-time 3D
7.
Three-dimensional vascular reconstruction
8.
Panoramic scan
9.
Spectral pulse Doppler
10.
US elastography (compression and shear wave)
11.
Other options (multislice view, volume computed tomography (CT), contrast US, etc.)
Our own experience is based on the analysis of examinations of 14,827 men and treatment of 4,847 patients with various breast pathologies (Fig. 4.2). The majority of cases of gynecomastia (4,500 men) were patients with diabetic gynecomastia who were treated in the endocrinology center of the Yaroslavl Railway Clinic of JSC “Russian Railways.” There were 180 patients with benign breast diseases and 225 men with breast trauma. More than 10,000 US examinations were performed in our clinic and the Yaroslavl Regional Oncologic Center.
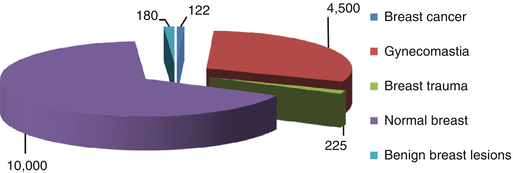

Fig. 4.2
Distribution of patients examined for breast abnormalities
Our experience is based on retrospective and prospective analysis of 122 cases of men with breast cancer treated in the Yaroslavl Regional Clinical Oncologic Center from 1993 to 2013. The mean age of the patients was 65.8 ± 8.6 years. Morphological examination of breast carcinoma in men demonstrated that all histological types observed in women were seen at practically the same incidence in men. Pathology of the material obtained from biopsies and after surgeries revealed the following types of breast carcinoma: ductal invasive carcinoma in 40.2 %, lobular invasive carcinoma in 9.9 %, tubular adenocarcinoma in 4.1 %, solid adenocarcinoma in 4.1 %, moderately differentiated adenocarcinoma in 3.3 %, serous adenocarcinoma in 1.6 %, squamous carcinoma in 1.6 %, leiomyosarcoma in 0.8 %, and unspecified carcinoma in 34.4 % of cases (Fig. 4.3).
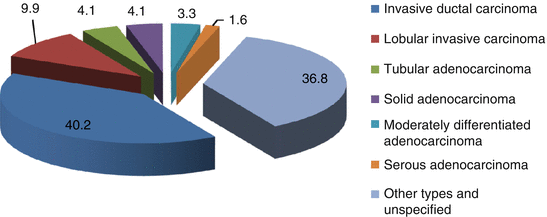

Fig. 4.3
Types of breast carcinoma
More than half of the patients were diagnosed with locally spread cancer. Metastases in lymph nodes were observed in 59 patients (48.3 %). More than four lymph nodes were affected in 16.4 % of patients. According to the TNM classification, the T1 stage of breast cancer was reported in 18.9 %, T2 in 44.3 %, T3 in 25.4 %, and T4 in 11.4 % of cases.
The majority of tumors had the second degree of malignancy (122 cases). Immunohistochemical study of samples for estrogen and progesterone receptors was performed in 85 % of patients, expression of Her2/neu in 65 %, and Ki-67 proliferation index in 57 % of patients. Seventy-seven percent of tumors appeared positive for both estrogen and progesterone receptors, whereas 3.3 % of tumors were negative.
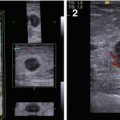
The clinical signs listed in the Table 4.1 were analyzed in men with breast tumors. Their expression substantially depended on the stage of malignancy. The US features of male breast lesions that are suspicious for cancer are decreased echodensity, eccentric location, heterogeneous structure, avascularity, and enlarged axillary lymph nodes (Table 4.2, Fig. 4.4). Our study proved that breast carcinoma in men has similar US signs as those observed in women (Sencha et al. 2011a, b, 2013a, b) (Fig. 4.5).
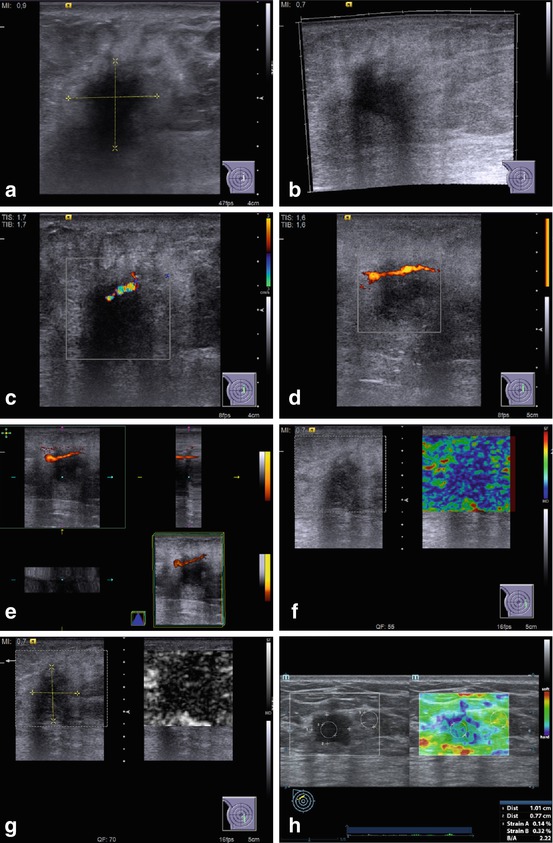
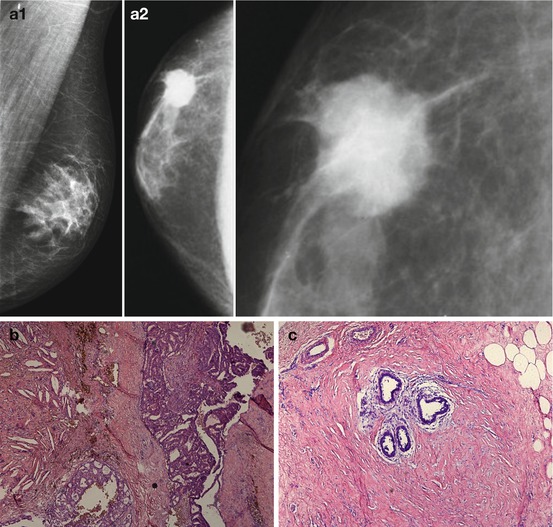
Table 4.1
Basic complaints and clinical signs in men with breast cancer
Clinical feature | Incidence (%) |
|---|---|
Palpable breast mass | 81.5 |
Eccentric location | 69.7 |
Discomfort in breast | 69.7 |
Deformation of the breast (especially in nipple and areola) | 65.6 |
Nipple retraction | 64.7 |
Pain | 34.4 |
Thickening and/or ulceration of the skin | 34.4 |
Breast edema | 34.4 |
Discharge from the nipple | 19.7 |
Lesions in the axillary area | 48.3 |
Absence of complaints | 3.3 |
Table 4.2
Basic US features of breast carcinoma in men
US regimen | US feature | Incidence (%) |
|---|---|---|
Grayscale US | Solid lesion in the breast | 98.4 |
Grayscale US | Eccentric location of a mass | 69.7 |
Grayscale US | Irregular shape of breast lesion | 60.65 |
Grayscale US | Rough margins | 69.7 |
Grayscale US | Indistinct contours | 56.55 |
Grayscale US | Decreased echodensity | 95.1 |
Grayscale US | Heterogeneous structure | 70.5 |
Grayscale US | Echogenic incorporations, microcalcifications | 15.6 |
Grayscale US | Anechoic component | 11.5 |
Grayscale US | Acoustic shadowing | 36.1 |
CDI, PDI, 3DPD | Avascularity | 55.7 |
Hypovascularity (1 color pixel) | 24.6 | |
Hypervascularity (2 and more color pixels) | 19.7 | |
CDI, PDI, 3DPD | Irregular distribution of vessels within the lesion, chaotic and disorganized vascular pattern, pathological transformation of vessels | 13.1 |
Compression US elastography | Intensive staining (red or blue), clearly distinct from the surrounding structures | 84.6 |
Uniform color of the mass | 88.5 | |
ARFI (VTQ) | Average shear-wave velocity within the lesion | 4.2 ± 0.032 m/s |
Strain ratio | Average value within lesion in relation to normal tissue | 2.8 ± 0.013 U |
Lymph node metastases | Axillary lymph nodes | 75.4 |
One sided | 59.0 | |
In combination with subclavial lymph nodes on the affected side | 10.65 | |
Remote metastases in organs and lymph nodes | 13.1 |

Fig. 4.4
US of breast carcinoma in a man. (a, b) Gray scale. (c) CDI. (d) PDI. (e) 3DPD. (f, g) US elastography. (h) Strain ratio

Fig. 4.5
Breast carcinoma in a man. (a) (1, 2) X-ray. (b) Photomicrograph of a histopathologic specimen (hematoxylin–eosin stain, original magnification, ×20). (c) ×100
The wide choice of US technologies and options with different frequencies of US scanning provide detailed images of breast structures and facilitate daily practice. High contrast and spatial resolution, and complex analysis of various US technologies permits easier and more effective data collection. Algorithms of automatic image optimization based on signal preprocessing allows balancing of the effects of tissue heterogeneity, to differentiate and suppress noise and artifacts.
4.3 Grayscale US
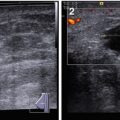
Grayscale (2D, B-mode) US is the principal US imaging modality for the diagnosis of breast diseases and breast cancer, in particular (Fig. 4.6). B-mode scanning permits assessment of the following US parameters of breast structure:
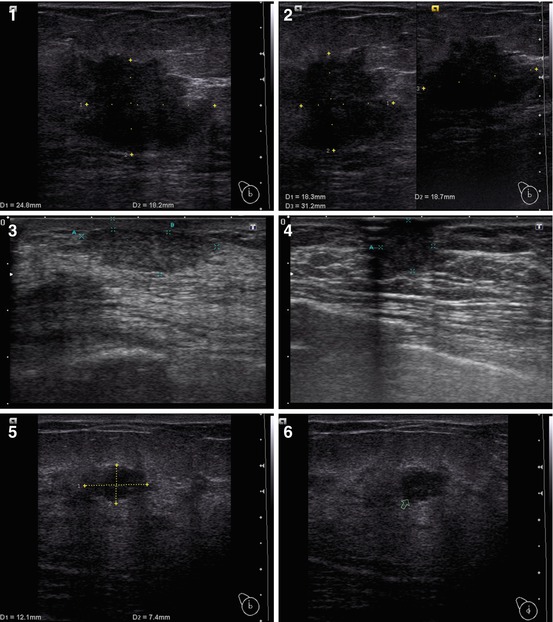
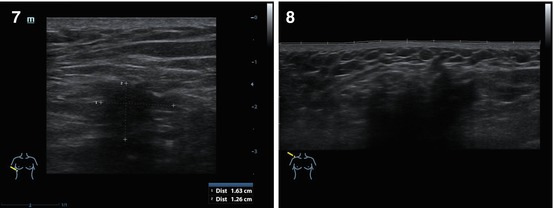


Fig. 4.6
(1–8) Nodular breast carcinoma. Grayscale US
Disturbance in the differentiation of tissues of the breast
Presence of one or several areas of local disturbance of the breast structure with clear or indistinct margins
Skin thickening
Indistinct border between the deep layer of derma and underlying structures
Presence and the expression of transverse and/or vertical hypoechoic tubular structures in the subcutaneous area
Rough connective tissue components of the breast
Presence of dilated lactiferous ducts
Grayscale imaging supplies basic information on the character of pathological process, thus decreasing the number of unspecified diagnoses. The wide range of histological types of tumoral growth invoke interest in searching for correlations between morphological and US structures.
Breast carcinoma in men, as well as in women, may exhibit nodular or diffuse (infiltrative) growth. The nodular type of breast cancer is primarily characterized by the presence of a lesion within the breast structure that can be revealed in grayscale mode or other US regimens. In our study, the nodular type of breast cancer was observed in 98.4 % of men (120 of 122 men). Sixty percent of tumors were located in the left breast. Tumors in the upper–outer quadrant were reported in 19.7 % of cases, in the upper–inner in 5.7 %, in the lower–inner in 3.3 %, and in the lower–outer in 1.6 %. Because of the small size of the male breast, the tumor, located in different quadrants of the breast, adjoined the areola edge in most cases. The tumor was bilateral in 3.3 % of men.
Nodular breast carcinoma has the following US features (Table 4.2):
Eccentric location of a mass in relation to the nipple
Decreased echodensity
Heterogeneity of the structure
Irregular shape
Rough and indistinct margins
Less often – calcifications within the lesion, fluid component, posterior changes in acoustic signal (Fig. 4.7)
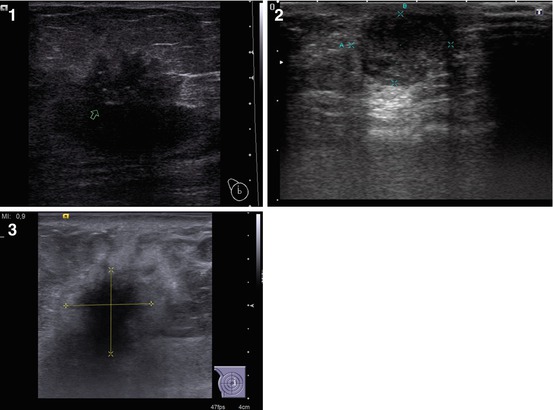
Fig. 4.7
(1–3) Nodular breast carcinoma. Calcifications within the tumor. Grayscale US
The location of a palpable breast tumor in men differs from the same in women. According to Zabolotskaya (2006), up to 50 % of all cancers in women originate from the upper–outer quadrant of the breast, 15 % are located in the upper–inner, 10 % in the lower–outer, 5 % in the lower–inner, and up to 17 % in the central area. In men, the central subareolar area is affected most often (59.7 %).
Considering that invasive lesions usually disorganize the normal location of layers of fat and connective tissue, malignant tumors can be slightly hypoechoic and have distinct contours. In the case of detection of calcifications and a heterogeneous echostructure with a spotted posterior shadow, the probability of a malignant tumor is very high.
Anatomical features of the male breast cause the most frequent location of cancer to be in the subareolar area. Tumors can be located under the nipple and merge into the skin. However, more often they have a slightly eccentric location. The last variant can be accompanied with an umbrella-shaped extrusion on the skin surface. It is very important to carefully examine the back edge of the lesion to assess its relation to the pectoral muscle because this influences the choice of treatment.
The lesion can demonstrate infiltrating or expansive growth. According to Ciatto et al. (1994), Marquet et al. (1995), Vetshev et al. (1997), and Trofimova (2002), US images of breast carcinomas are quite variable. The basic criteria of breast cancer with grayscale US are the following: irregular shape of the lesion, heterogeneity of the echostructure, rough indistinct contours, and posterior acoustic shadows of various intensities often emerging from the posterior or lateral aspects of the tumor. While analyzing the shape of a lesion in grayscale mode, attention should be paid to the orientation of the long axis of a malignant breast lesion toward the skin with the prevalence of anteroposterior size.
There is no common opinion in the literature regarding microcalcifications in male breast cancer and their value for diagnosis I. Rosen and H. Nadel (1966) described microcalcifications on mammograms of a man with breast carcinoma. The analysis of publications at that time allowed the authors to say that it was the first case of a preoperative radiological diagnosis of microcalcifications in breast cancer in men. L. Kalisher and R. Peyster (1975) considered that the absence of microcalcifications was a typical feature of breast carcinoma in men. Other researchers also observed this sign in men, however, at an extremely rare incidence of 3–5 %. Some authors diagnosed calcifications in 15 % of men with breast cancer, but pointed out their specific feature. Patients had not only multiple microcalcifications in groups but also large coarse calcifications similar to those observed in fibroadenoma in women (Ostrovskaya et al. 1988). In males, as opposed to women, there are no cyclic hormonal processes that define breast status. This may explain the fact that men’s breasts in benign conditions do not contain calcifications of vessels, ducts, foci of gynecomastia, or benign tumors. The detection of calcifications of any character on a male mammogram is a bad sign. It is necessary to note that calcifications in men, unlike in women, are always observed in the tumoral mass. Haylenko et al. (2005) reported microcalcifications in 33 % of cases of breast cancer and note that this sign has a high specificity in women. According to Zabolotskaya (2006), microcalcifications are reported in 65 % of intraductal breast carcinomas in women.
Breast carcinoma can be bilateral in 3–15 % of cases. The cancer within the second breast can arise simultaneously with the tumor of the first breast (Rozhkova 1993). Multicentric cancer is observed in 18 % of women, and synchronous affection of the second breast is detected in 43 % of cases. Male breast cancer exhibits similar figures.
In some cases, grayscale US images of breast carcinoma in men can look like gynecomastia without typical malignant features (Dickson 2011; Sencha et al. 2011a, b). Other authors consider that tumoral diseases of the male breast can be hardly differentiated with US from true gynecomastia (Zabolotskaya and Zabolotsky 2010). Any doubt about the benign character of a lesion should be followed by puncture biopsy.
Breast US has some limitations in the detection of lesions against adipose infiltration and in the assessment of ductal extension of the tumor. This is of particular concern for men who have had surgery for gynecomastia. Retromammary tumors and lesions smaller than 1 cm in size may be left undetected. One significant disadvantage of US is a high dependency on the operator.
In our study, the infiltrative type of breast carcinoma was observed in 1.6 % of men (2 of 122 men). Infiltrative breast cancer is often characterized with grayscale US by the following features:
Thickening of the skin
Increase in echodensity of the subcutaneous adipose layer
Disorganization of its structure
Asymmetric dilation of lactiferous ducts and lymph vessels
Enlargement of regional lymph nodes
Grainy irregular color pattern with US elastography
The absence of a lesion within the breast often creates difficulties in the differential diagnosis of the infiltrative type of breast carcinoma in men (as well as in women).
4.4 Tissue Harmonic US
Tissue harmonic US is based on an algorithm to detect the harmonic components of US waves induced in tissues with basic US impulses (Fig. 4.8). The option is usually incorporated into ordinary scanners and uses conventional US probes. It permits the detection of US signs of breast carcinoma that are more exact (the quality of imaging subjectively improves in 18.7 % of cases), mainly at the expense of accurate definition of the contours and heterogeneity of the lesion with specification of the presence and location of calcifications. The option is especially effective in the assessment of large (>30 mm) or small (<5 mm) lesions; however, it does not have a high value in the differential diagnosis of breast carcinoma in men.
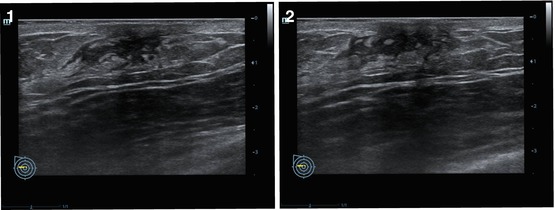

Fig. 4.8
(1, 2) Breast carcinoma. Tissue harmonic imaging
4.5 Adaptive Coloring
The technology of adaptive coloring uses a color scale in place of the gray scale used in conventional US. The intensity of color depends on the power of the obtained US signal (Fig. 4.9). Inversion of the color of the image is possible. Adaptive coloring is applied as a software option for color US systems and does not require hardware modifications. The option is effective in combination with the grayscale mode for the detection of breast abnormalities (often isoechoic), characterization of its contours, and effects of posterior artifacts, especially in small-sized lesions (<0.5 cm). This technology is seldom used to diagnose breast abnormalities.
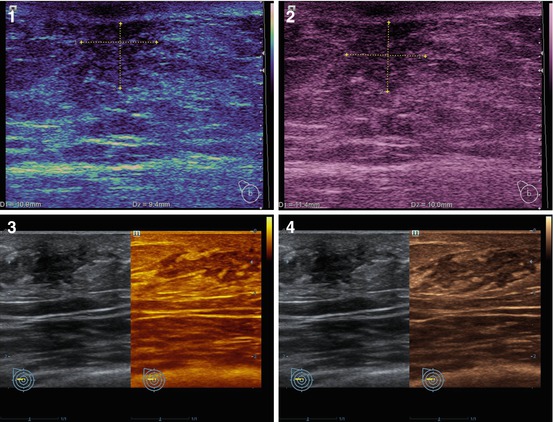

Fig. 4.9
(1–4) Breast cancer. Adaptive coloring
4.6 Color and Power Doppler Imaging
Color Doppler imaging (CDI) is an US technology of blood flow imaging based on registration of velocities of blood flow in the area of interest with further coding with different colors and superimposition on a 2D grayscale image. The modality is available on all modern scanners and uses the same probes as conventional grayscale US. Modern US equipment with Doppler imaging permits imaging of blood flow in arteries and veins of calibers greater than 2 mm. The majority of researchers and practitioners agree about the great value of CDI for the diagnosis of malignant breast lesions.
CDI normally detects poor vascular patterns in breast tissue. The color pattern generally is represented by individual color points, which are distributed in a relatively regular way among all quadrants of the breast (Kharchenko et al. 1993a, b; Sandrikov and Fisenko 1998). Several methods to make color and power Doppler imaging more objective were suggested, such as calculation of color pixel density. According to Folkman (1995), one significant feature of breast carcinoma is vascular asymmetry.
Tumor growth is often accompanied with neovascularization and development of an independent pathological vascular rete. Neoangiogenesis of a malignant tumor results in vascular architectonics that differ from benign lesions and physiological breast changes. According to Holcombe et al. (1995), a large number of vessels within a malignant breast lesion suggests a higher stage and possible metastases in axillary lymph nodes.
Our study revealed that the nodular type of breast carcinoma in men is predominantly avascular with CDI (55.7 % cases), with no vessels within the tumor. One vascular pixel was interpreted as a hypovascularization and was reported in 24.6 % of men with breast carcinoma. Two and more color pixels (hypervascularization) were defined in 19.7 % of patients. Vascular pattern and neoangiogenesis in men with breast malignancies was significantly poorer than in women with the same pathology. We consider one and more vascular pixels within the lesion suspicious for cancer (Fig. 4.10).
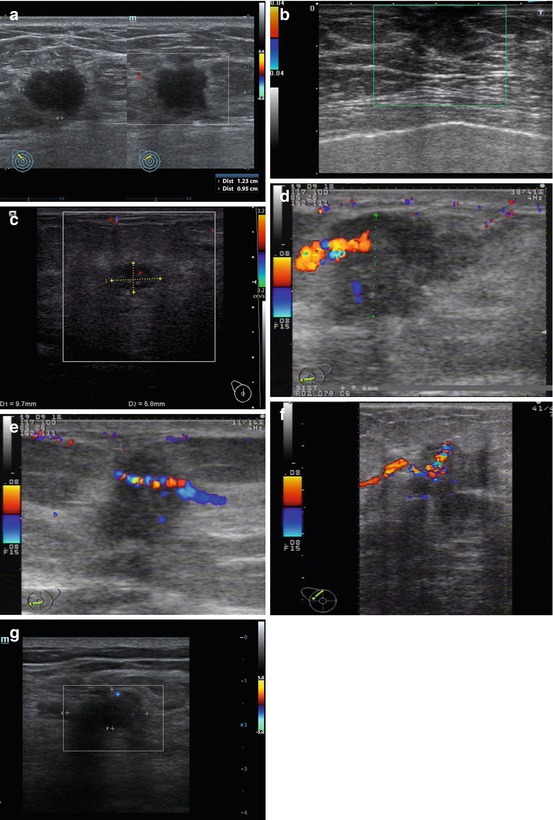

Fig. 4.10
Breast carcinoma. Sonograms. (a–c) CDI. (d) Radial course of the vessel. (e–g) Penetrating vessels in a tumor
Madjar et al. (1995) suggested counting all vessels detected within the nodule for quantitative assessment of the vascularity of breast lesions. According to their data, benign lesions demonstrated 2 arteries on the average, whereas malignant lesions demonstrated up to 11 arteries. Sohn et al. (1997) report correlation between vascularization and size of the tumor. Fiedler et al. (1996) consider that CDI is effective only in tumors larger than 1–2 cm in size (blood flow was identified in 90 % of cases, whereas in tumors <1 cm, in only 41.7 %). According to Сhao (1999), the average size of avascular breast neoplasms was 1.9 ± 0.1 cm, and vascularized tumors had an average size of 2.7 ± 0.1 cm. Sohn et al. (1997) consider that the mentioned methods of scoring are too labor intensive and are not reliable in practice because of high operator and scanner dependency.
According to Madjar et al. (1995), the vessels in a malignant breast neoplasm can be detected in 43–100 % of instances with 5–14 arteries (8–11 arteries on average) identified within the tumor. Madjar et al. (1995) report that the presence of two and more vessels within a tumor is a typical sign of cancer. According to Ivanova (2006), only infiltrative cancer is vascularized in 100 % of cases. It has specific characteristics with pulsed-wave Doppler, such as the absence of a diastolic component and arteriovenous shunts. Trufanov et al. (2009) report that the majority of malignant breast tumors are well vascularized and exhibit intranodular (72.6 %) or mixed (13.8 %) types of vascular patterns.
Cosgrove et al. (1993) differentiate three types of blood flow within a lesion: perinodular, intranodular, and mixed. Lesions are often divided into hypervascular (with multiple arterial and venous vessels), hypovascular (with two–three color pixels), and avascular (without color pixels) (Kharchenko et al. 1999). Nadareishvili (2002) suggested dividing Doppler signals in lesion structures into four types according to their shape: individual, linear, chaotic, and branching. Depending on the localization, the tumoral blood flow is divided into three groups: central (up to 6.4 %), peripheral (25.9 %), and mixed (67.7 %).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree