Chapter 7 Ultrasound of Polyneuropathies
Peripheral nerve ultrasonography is in evolution and its uses are expanding. Nearly two decades ago, the first papers were published on ultrasonographic abnormalities in carpal tunnel syndrome.1,2 After initial skepticism and numerous studies, ultrasonography is becoming widely accepted as a means of evaluating structural abnormalities of the nerve including entrapment, tumors, and transection. Used in conjunction with electrodiagnostic studies, it has the ability to improve patient care. The role of ultrasonography in the assessment of polyneuropathy remains less well defined and parallels the beginnings of research on nerve entrapment syndromes. More rigorous studies are needed, but case reports and case series provide ample hope for the future. In this chapter, the existing literature on ultrasonography in neuropathy is presented along with its implications for diagnosis, treatment, and prognosis.
A Few Words on Getting Started
Standards of Measurement
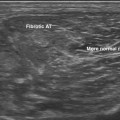
Optimal sites for measurement of the nerves in polyneuropathy have not been established. A commonly accepted method is to scan the length of the nerve prior to making any measurements. The median and ulnar nerves are most helpful because it is easy to visualize their entire course. By scanning the entire length of the nerve, areas of abnormality are quickly identified and measurements can be made at those sites. Routine measurements are then made at predesignated anatomic landmarks for each nerve (e.g., the antecubital fossa for the median nerve). Cross-sectional area is considered the most reliable measure of nerve size,3 but longitudinal views of the nerve are also useful for demonstrating areas of focal enlargement or compression. All measurements listed in this chapter provide the cross-sectional area in mm2, unless otherwise indicated.
It is necessary that each laboratory establish its own standards and normal values for measuring nerve area and/or diameter. This is no different than the existing recommendations for laboratories performing nerve conduction studies. Published values serve as helpful guideposts but may have been obtained using different techniques, different equipment, and different patient populations. If published values are to be used, clinicians should use the same methods as listed in the relevant publication and select a sample control group that might be similar to their own patients to ensure its validity.
Although not specifically mentioned in many articles or texts, it is important to consider age, gender, height, and weight when defining abnormalities in peripheral nerve size. Some studies have suggested relationships between these factors and nerve cross-sectional area.4,5 Although there is currently no well-established method to adjust for these factors, it is worth considering that male gender, increasing height, and increasing body mass index may be associated with larger nerve cross-sectional area (although the influence of gender may be a function of height and weight). The role of age is less clear at this time and there are conflicting opinions, although there has been some suggestion that increasing age correlates with increasing nerve size.6,7
In addition to nerve size, other aspects of nerve appearance should be documented. Echogenicity and vascularity can be easily assessed in a qualitative manner. Quantitative measures of echogenicity have been described, but a standard means of reporting these findings has not been established at this time.8–10
Immune-Mediated Neuropathies
Chronic Inflammatory Demyelinating Polyneuropathy
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an autoimmune disorder of the peripheral nervous system and results in sensory and motor impairment that may have a fluctuating course. It is typically responsive to treatment with corticosteroids, intravenous immunoglobulin, or plasmapheresis.11,12 However, the degree of recovery is often limited by the extent of secondary axonal loss. Early treatment is crucial in preventing long-term disability, but accurate identification of the disorder in its earliest stages may be difficult. The diagnosis is based on a combination of clinical and electrodiagnostic findings. Published research criteria for definite diagnosis of CIDP require rather stringent electrodiagnostic abnormalities (e.g., rigorously defined conduction block) in at least two motor nerves.13,14 These abnormalities are often absent in some stages of disease, and any technology that permits earlier diagnosis and treatment stands to significantly improve patient care.15,16
The first description of nerve ultrasonography findings in CIDP was published in 2000.17 The authors examined a single patient with a 3-year history of CIDP. Her diagnosis was supported by the clinical presentation, an elevated cerebrospinal fluid (CSF) protein level, abnormal nerve conduction studies, and nerve biopsy. She was experiencing recurrent weakness at the time of the examination and happened to undergo ultrasonography using a 7-MHz linear array transducer as part of an evaluation for goiter. No goiter was found, but marked enlargement of the bilateral brachial plexus was noted. This finding led to further examination of the peripheral nerves. Several, but not all, were noted to be enlarged. Cross-sectional areas were not provided in the study, but it is mentioned that the median nerve was 5 mm in diameter on longitudinal images. The nerve hypertrophy was not unexpected and had previously been reported with magnetic resonance imaging (MRI),18–20 and correlates with the classic histologic “onion bulb” appearance seen in nerves affected by CIDP.21 The onion bulb results from episodes of repeated demyelination and remyelination, creating areas of nerve enlargement.
Following this initial publication, several years passed before the topic was again addressed. In 2004, Matsuoka and colleagues published a study examining the cervical nerve roots in 13 patients with CIDP and 35 control subjects using a 7.5-MHz linear array transducer.22 The C5-7 nerve roots were examined in both groups, and normal values for diameter were established using the group of healthy controls. The authors demonstrated the presence of cervical root hypertrophy in 9 of 13 CIDP patients and noted the enlargement had a direct correlation with CSF protein levels. Based on this evidence, the authors postulated that ultrasonography screening for nerve root hypertrophy might play a role in the diagnosis of CIDP.
A more comprehensive look at the ultrasonography findings in CIDP was recently published in 2009. Zaidman and colleagues included 36 individuals with CIDP as part of a larger study on ultrasonography and neuropathy.4 The median and ulnar nerves were imaged and cross-sectional area measured at predetermined sites in the upper extremities. Patients with CIDP were found to have diffuse nerve enlargement, estimated to be 2.3-fold larger than that seen in the control group. There was a direct correlation between duration of CIDP and nerve size, and an inverse correlation was noted between cross-sectional area and conduction velocity.
Another development is the possible use of ultrasonography for identification of conduction blocks in CIDP. Conduction blocks are often difficult to demonstrate when present in proximal nerve segments, a factor that may lead to delays in diagnosis and treatment. Three reports describe focal nerve enlargement at the site of electrodiagnostic conduction blocks in two patients with CIDP.23–25 Two of the reports provide images of upper extremity nerves (median, ulnar), with nerve enlargement clearly demonstrated in longitudinal section.24,25 Although this seems to be a promising development, it is worth noting that Zaidman and colleagues did not describe a similar phenomenon.4 Additionally, one of the reports notes that the focal nerve enlargement did not resolve when the patient clinically improved.25 Given prior data that nerve size correlates with duration of CIDP,4 it may be that areas of focal enlargement persist indefinitely. This factor may limit the use of ultrasonography in monitoring the response to therapy.
Clinical Application—CIDP, Case 1
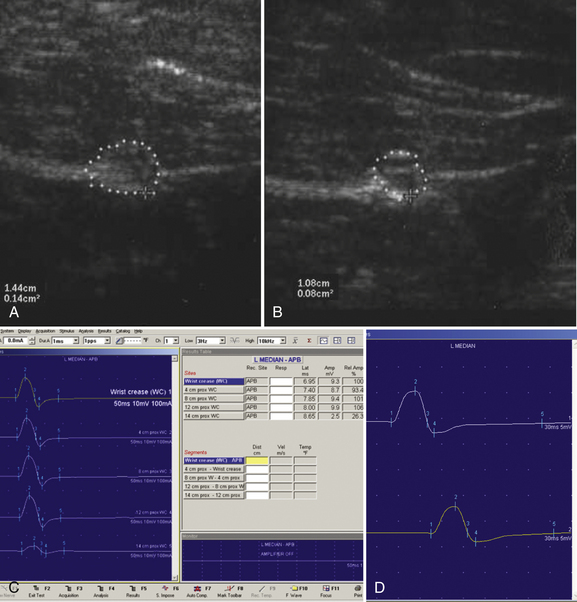
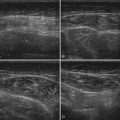
A 20-year-old woman presented with a 6-week history of paresthesias in right digits IV and V, associated with a decline in grip strength. Clinical examination revealed Medical Research Council (MRC) grade 4/5 weakness of the first dorsal interosseous and abductor digiti minimi. There was no sensory loss. Reflexes were absent throughout the upper and lower extremities. Electrodiagnostic studies demonstrated right ulnar neuropathy at the elbow, along with conduction block in the left median nerve (Fig. 7.1C).
Ultrasonography of both upper extremities revealed focal enlargement of the right ulnar nerve at the elbow that could not be differentiated from an entrapment neuropathy. The right median and left ulnar nerves were normal. The left median nerve demonstrated focal enlargement 14 cm proximal to the distal wrist crease—the site of electrodiagnostic conduction block (Fig. 7.1A). She was treated with intravenous immunoglobulin (IVIG) and returned 4 weeks later. There was resolution of the left median conduction block and resolution of the ultrasonographic changes (Fig. 7.1B and D). In this patient, ultrasonography has subsequently been used to monitor response to treatment.
Clinical Application—CIDP, Case 2
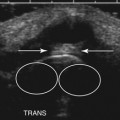
Ultrasonography of the right median nerve showed cross-sectional area of 8 mm2 from the wrist to just above the antecubital fossa (Fig. 7.2A). At 7 cm proximal to the elbow crease the median nerve enlarged to 21 mm2 (Fig. 7.2B and C) and then returned to its previous size of 9 mm2 over a length of 3 cm. This area of focal enlargement provided supportive evidence of CIDP in a case in which NCS were inconclusive. The patient was treated with IVIG and noted rapid improvement in lower extremity strength. He did not return to the laboratory for further testing.
Acute Inflammatory Polyradiculoneuropathy
Acute inflammatory demyelinating polyradiculoneuropathy, or Guillain-Barré syndrome, is the most common cause of nontraumatic paralysis worldwide, affecting 1 to 2 persons per 100,000 each year.26 An immune-mediated disorder, it results in acute onset of ascending paresthesias, weakness, and loss of reflexes. It is one of the few neuromuscular emergencies, and early identification is important because respiratory weakness may ensue. The diagnosis is primarily based on the clinical presentation because electrodiagnostic changes, and CSF cytoalbuminologic dissociation may not be present within the first few days of illness.27
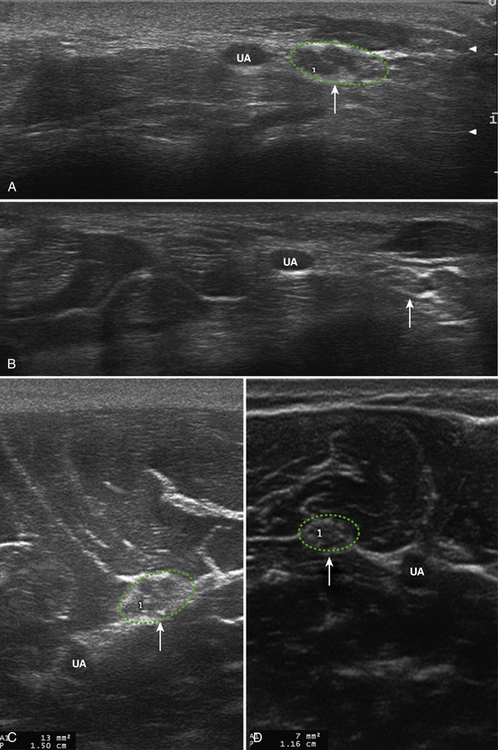
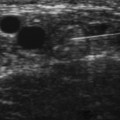
Little is known regarding the ultrasonography changes of acute inflammatory demyelinating polyradiculoneuropathy. Zaidman provides the available published information.4 Seventeen subjects with acute inflammatory demyelinating polyradiculoneuropathy were imaged, with 13 of 17 being imaged within 4 weeks of onset. The ulnar and median nerves were imaged in all and found to be 1.4-fold enlarged as compared with controls. There was no correlation between this enlargement and any electrodiagnostic parameter. Figure 7.3 shows diffuse enlargement of the ulnar nerve in a patient with acute inflammatory demyelinating polyradiculoneuropathy at approximately 2 weeks into the illness. Median and radial nerves were also found to be diffusely enlarged, but are not pictured.
Multifocal Motor Neuropathy
Multifocal motor neuropathy is a demyelinating polyneuropathy that affects only motor nerves. It is associated with the presence of anti-GM1 antibodies, but the diagnosis rests on the clinical examination and electrodiagnostic findings. As with CIDP, conduction block is a sentinel feature, but often difficult to demonstrate when present in the proximal segment of nerves. Histologic evaluation of affected multifocal motor neuropathy nerves has demonstrated demyelination, along with edema and rare “onion bulb” formation.28–31 Nerve enlargement/hypertrophy is not an unexpected finding. As with CIDP, ultrasonography may have a promising role in both the diagnosis and monitoring response to therapy.
Beekman and coworkers published the only existing article on ultrasonography of multifocal motor neuropathy.32
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree