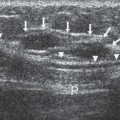
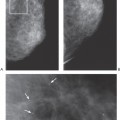
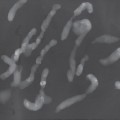
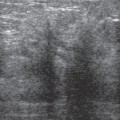
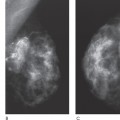
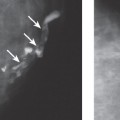
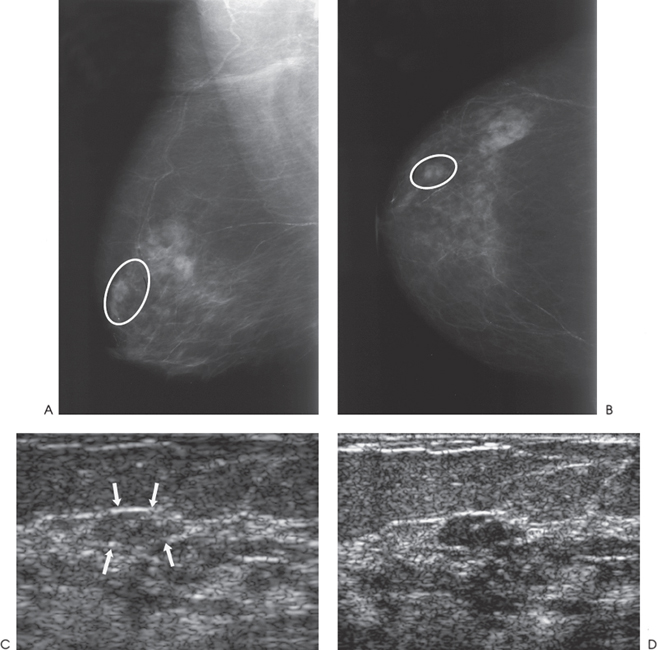
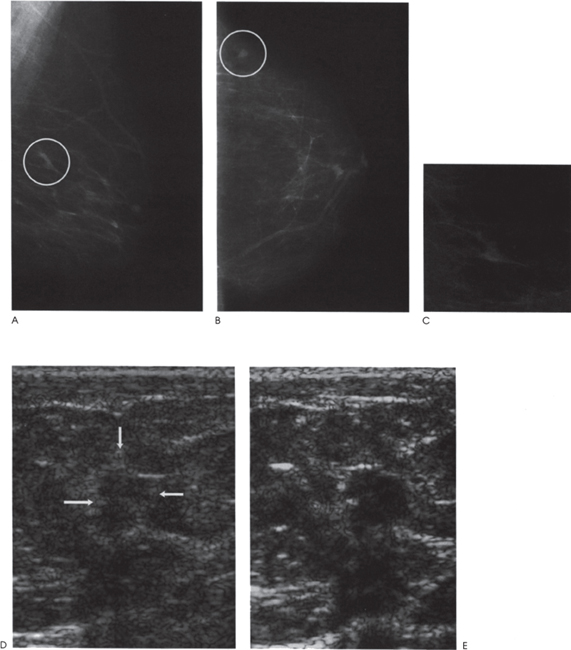
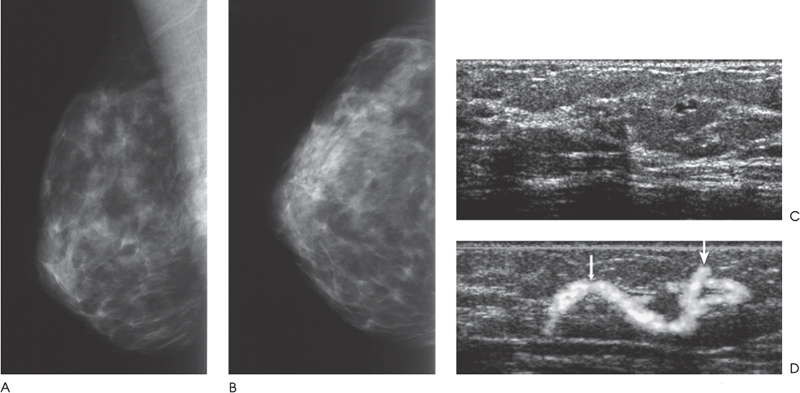
Chapter 2 The main applications of ultrasound are identification of a palpable breast lump and clarification of a confusing mammographic finding. In the past, a common reason that sonographic applications were limited was that the ultrasound examiner could not cross correlate the physical examination or mammographic findings with the sonographic information. This inability to cross correlate the ultrasound examination with the physical examination or mammographic asymmetry is frustrating and leads to long, sonographic examinations. In order to develop the ability to cross correlate sonography with mammography, one should have optimal sonographic equipment and technique, be able to palpate breast masses, be familiar with normal mammographic and sonographic breast anatomy, recognize mammographic and sonographic signs of malignancy, and apply anatomic knowledge to sonographic scanning. Ultrasound Equipment and Technique Equipment In the past, breast ultrasound has required the least sophisticated equipment because there were few sonographic breast applications and these applications required only simple equipment. However, if one wishes not only to have a high rate of localizing solid masses as well as an optimal image to characterize the mass, then one needs a sophisticated machine. In this book, when I refer to high frequency, I am generally referring to imaging with frequencies equal or greater than 10 MHz. To have maximal flexibility, one should have a machine that has linear transducers with frequencies ranging from 7 to 15 MHz. High frequency is important as many breast structures are small. An important aspect of the technical advancement of mammography is the improvement of spatial resolution. Because of high mammographic resolution, mammographers are identifying smaller and subtler abnormalities. To be an effective adjunctive test, high sonographic spatial resolution is needed to clarify these subtle findings. Because normal breast structures such as ducts and terminal duct lobular units are small, high spatial resolution allows the ultrasound examiner to quickly recognize normal breast architecture and identify small malignant masses in the ductal system. Besides being able to identify smaller malignancies, high spatial resolution improves the sonographic image so one can better characterize masses. This improved image is similar to the visual effect experienced by a nearsighted person who starts wearing glasses. The image is sharper, and subtle or smaller details are clearer (Fig. 2–1; also see Section 4 Case 57). For breast malignancies, the most important information produced by high-frequency ultrasound is the improved ability to see the margin of the mass and identify secondary signs of malignancy such as spiculation or architectural distortion. Occasionally, one can trace the path of the malignancy through the ductal system and identify the extent of the disease better than mammography (see Section 10, Cases 200 and 201). Imaging Techniques Besides the availability of high-frequency transducers, sophisticated ultrasound machines have more technical factors that can be manipulated to improve imaging quality. For breast ultrasound, an important factor in image quality is being able to obtain excellent contrast resolution. The reason contrast resolution is important is that one must be able to distinguish a variety of masses from the background parenchyma. When the breast is fatty, focal masses such as complex cysts, lymph nodes, fibroadenomas, and cancers may be difficult to identify. When the breast is dense, fat necrosis and surgical or radial scars may be hard to locate. Many factors can improve contrast resolution. High spatial resolution produced by high-frequency imaging improves contrast resolution because the assignment of gray shades is more precise. Reducing the dynamic range may improve contrast resolution as this method exaggerates differences between the gray shades of structures. A variety of proprietary postprocessing programs improve contrast resolution by enhancement of specified gray shades on a point-by-point basis. One type of proprietary postprocessing program improves contrast resolution by enhancement of specified gray shades on a region-by-region basis. One should consult the manufacturer of one’s equipment to learn which imaging techniques are available (Fig. 2–2; also see Section 3 Case 18). Figure 2–1. (A). Right MLO mammogram. (B). Right CC mammogram. (A,B). In the upper outer right breast there is a lobulated density (circled). (C). Right radial breast sonogram: A 5 MHz transducer does not clearly demonstrate the lobulated mass (arrows). The inadequate size and contrast resolution results in poor definition of the mass. (D). Right radial breast sonogram: An 8 MHz transducer improves the definition of the mass. This result allows an observer to confidently localize this mass. This mass is a fibroadenoma. Besides adjusting image contrast, one should be aware of software methods to optimize resolution. These methods include increasing the line density of the image, increasing the persistence, and adjusting the focal zones. The main disadvantage of these methods is a slower frame rate. If one is merely characterizing a lesion, a slower frame rate may not be a problem. However, the slower frame rate may be disconcerting with real-time imaging of interventional procedures. Color or power Doppler is a useful method to quickly assess vascularity. Breast vascularity is low, so one should be aware of methods to optimize the color or power Doppler. Generally, this means that one is using a color or power Doppler frequency slightly lower than the gray scale frequency and the focal zone adjusted at the correct depth. The filter and scale should be low. The Doppler gain is optimized by initially increasing the gain until the entire screen is filled with color and then by slowly reducing the gain until the color appears only within pulsating vascular structures. If no color is detected using these methods, then the sample volume size should be increased. Increasing the sample volume size reduces color resolution. The color may “bleed” and be demonstrated outside of the vessel walls. Color or power Doppler is useful to delineate vessels or highly vascular structures such as arteriovenous malformations. This Doppler technique is also useful to clarify whether a hypoechoic or anechoic mass is cystic or solid (Fig. 2–3; also see Section 3 Case 32). Figure 2–2. (A). Left MLO mammogram. (B). Left CC mammogram. (C). Left MLO spot magnification mammogram. (A–C). In the left upper outer quadrant, there is a small irregular mass (circle). (D). Left antiradial (8 MHz) breast sonogram: Sonographic examination of the mammographic mass with a low-contrast technique poorly demonstrates the mass (arrows). The hypoechogenicity of the mass blends into the surrounding fat. (E). Left antiradial (8 MHz) breast sonogram: Sonographic examination of the same location as Figure 2–2D with high-contrast technique greatly improves the conspicuity of the mass. This mass is a mucinous carcinoma. Figure 2–3. (A). Right MLO mammogram. (B). Right CC mammogram. (A,B). The patient and the breast surgeon have identified some small palpable lumps at the 9:00 position of the right breast. The lumps are arranged in a linear pattern. The left breast has similar lumps that are less conspicuous. Bilateral mammograms are normal. (C). Right radial breast sonogram: Gray scale sonographic examination of the palpable lumps shows normal tissue. (D). Right radial breast sonogram: Color Doppler examination of the palpable lumps demonstrates that the lumps are due to the lateral blood vessels of the breast. Each lump corresponds to the superficial curve of the blood vessel (arrows) (see Color Plate 2–3D). Dynamic clips are useful to document vascularity and to demonstrate the spatial relationship of multiple lesions. Dynamic clips are the ideal method to show color flow in pseudoaneurysms or intravenous contrast enhancement of solid masses. Until high-resolution three-dimensional (3-D) imaging is universally available, dynamic clips are an excellent way to demonstrate the relationship of multiple cysts to a solid mass or to show debris or calcifications moving within a complex cyst. Wide field of view compound imaging is sometimes useful to document larger masses or the relationship of multiple masses in the same plane. The wider field of view provides observers with more landmarks so cross correlation with mammography and magnetic resonance imaging (MRI) may be easier. Newer sonographic techniques that may have more applications in the future include 3D imaging and harmonic imaging. Like compound imaging, 3-D imaging may produce a wide field of view that would be similar to a mammogram or magnetic resonance image. In the future, 3-D imaging may also allow surgeons and patients to better appreciate the location and size of sonographic findings and facilitate surgical planning. Harmonic imaging may improve image resolution and increase both gray scale and color Doppler sensitivity for intravenous sonographic contrast agents. These contrast agents may improve both vascular and gray scale characterization of masses. Approach to a Palpable Mass In many breast centers, palpable masses are the most common reason for a breast sonogram. Therefore, it is important that sonographic breast imagers learn how to palpate breasts. Usually, the patient will be able to identify the palpable lump. When the patient locates the lump, the breast imager should confirm the presence of the lump by palpating the area identified by the patient. Even if the lump is obvious, the imager should scan the lump and reconfirm the location of the lump by moving a finger into the scan plane. If the imager cannot detect the lump or if the patient is not sure of the exact location of the lump, then palpation of the entire quadrant is useful. By palpating a larger area, one is able to detect asymmetries within the region. Finally, if palpating the quadrant isn’t helpful, then one may palpate the comparable area in the opposite breast. Commonly, the parenchymal pattern of patients is symmetric, so the physical exam is also symmetric. By palpating the corresponding contralateral quadrant, one may detect abnormal asymmetries. This technique is particularly useful with malignancies that are commonly difficult to feel such as lobular carcinoma. Cross Correlation of Sonographic and Mammographic Image To accurately, efficiently, and confidently sonographically identify a mammographic abnormality, the personnel who perform the breast sonographic examination should be familiar with mammographic imaging. Furthermore, the ultrasound examiner should be able to review the mammogram and identify internal landmarks that can be cross correlated with the ultrasound. Finally, by confirming the mammographic landmarks sonographically, the examiner should be able to pinpoint the location of the mammographic abnormality in the breast with ultrasound and consequently be able to explain the etiology of the puzzling mammographic finding. Unfortunately, sometimes the ultrasound examiner does not attempt to closely cross correlate anatomically the ultrasound examination with the mammogram. In some cases, the examiner does not attempt to correlate the exams because he or she does not routinely interpret mammograms and is uncomfortable reviewing mammograms. However, more common reasons for lack of close cross correlation include the following: (1) The sonographic image has a small field of view compared with the mammographic global field of view. (2) The patient position for an ultrasound examination is completely different than the position for the mammographic examination. Therefore, the position of a breast mass for these exams appears extremely different. (3) Even if one places the patient in the same position, the difference in technology between ultrasound and mammography creates different orientations of tissue visualization. (4) Unlike other organs, the breast does not have a uniform or constant normal anatomy. The breasts of different individuals have different breast architecture. Furthermore, some individuals have a right breast that exhibits a pattern different from the left breast. Finally, the breasts of many individuals change with age.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree