Screening mammography aims to identify small, node-negative breast cancers when they are still curable while maintaining an acceptable range of false-positive recalls and biopsies. The mammography audit is a powerful tool to help radiologists understand their performance with respect to that goal. This article defines audit terms and describes how to use collected and derived data to perform a mammography audit. Accepted benchmarks are discussed as well as their applicability to radiologists and breast imaging practices in the United States. Special considerations regarding volumes and radiologist characteristics are explored, because these factors may affect audit results.
Key points
- •
To be compliant with the Mammography Quality Standards Act, the US Food and Drug Administration mandates a medical outcomes audit program for each facility on an annual basis.
- •
The audit required by the US Food and Drug Administration differs from the audit recommendations from the American College of Radiology.
- •
Separate audit data should be collected for screening mammograms and diagnostic mammograms because the performance benchmarks differ.
- •
To gauge performance, use multiple audit metrics rather than a single metric.
- •
Double reading, increasing reading volumes, obtaining all available prior examinations, reviewing call backs, and reviewing false-negative cases may improve performance.
Introduction
Mammography remains the gold standard for breast cancer screening and has been proven to reduce breast cancer mortality; however, there is variability in the performance of mammography across different facilities and radiologists. To establish uniform quality standards, the Mammography Quality Standards Act (MQSA) was enacted in 1992. The US Food and Drug Administration (FDA) issued the MQSA final regulations, under which mammography facilities are currently regulated. The MQSA requires each mammography facility to meet certain quality standards, maintain certification and accreditation, and undergo regular inspections and surveys.
As a part of the quality assurance process, a medical outcomes audit is required at least once every 12 months at each mammography facility. Per the MQSA, there should be a system in place to track “positive” mammograms, collect pathology results from biopsies performed, correlate the pathology results with the final assessment category, review any known false-negative mammograms, and assess medical outcomes audit data. , At a minimum, facilities must track “positive” mammograms interpreted as suspicious or highly suggestive of malignancy. Individual as well as aggregate performance parameters should be calculated and a designated interpreting physician, known as the lead interpreting physician, should review the data and be responsible for documenting audit results and notifying the other radiologists of their results.
The American College of Radiology (ACR) asserts that more complex auditing than required by MQSA should be performed to determine acceptable clinical performance. The ACR has published the procedures for performing a basic and more complete audit in the Breast Imaging Reporting and Data Systems (BI-RADS) Atlas. The audit allows assessment of strengths and weaknesses when compared with nationally recognized benchmarks. ,
This article defines the ACR mammography audit terminology, reviews the basic and more complete mammography audit, and discusses how to assess your practice and individual performance relative to published performance benchmarks.
Audit terms and definitions
This section reviews the basic audit terminology and provides pertinent examples.
Screening Mammography Versus Diagnostic Mammography
Positive screening examination
Additional diagnostic imaging is recommended or a tissue diagnosis a
a Tissue diagnosis . Pathologic diagnosis made by an interventional procedure (fine-needle aspiration, core biopsy, or incisional or excisional biopsy).
is recommended. BI-RADS assessments of 3, 4, or 5 at screening are highly discouraged ( Box 1 ) ( Table 1 ). Note that a positive screening mammogram as defined by the ACR is different than the definition of a positive examination in the MQSA Final Rule.
Screening mammogram
Performed to detect occult breast cancer in asymptomatic patients
Diagnostic mammogram
Indications include
- i.
Call back after screening mammogram
- ii.
Clinical signs or symptoms
- iii.
Follow-up imaging of a probably benign finding
- iv.
Response evaluation after neoadjuvant treatment for breast cancer
- i.
Asymptomatic women with a personal history of breast cancer or benign biopsy may undergo annual diagnostic rather than screening mammograms. For audit purposes, those examinations should be included in the screening group.
| Positive Screening Examination | Positive Diagnostic Examination |
|---|---|
| BI-RADS 0 | BI-RADS 0 b |
| BI-RADS 3, 4, 5 a | BI-RADS 4 and 5 |
a Use of BI-RADS 3, 4, and 5 on a screening mammogram is highly discouraged .
b Use of BI-RADS 0 at diagnostic mammography is discouraged . An addendum issuing a final assessment should be performed. For the rare instance in which a BI-RADS 0 assessment remains for a diagnostic mammogram, the examination is coded positive.
Positive diagnostic examination
Tissue diagnosis is recommended (see Table 1 ).
Negative screening examination
An examination evaluated as negative or benign (BI-RADS 1 or 2).
Negative diagnostic examination
A tissue diagnosis was not recommended. The examination was assessed as negative (BI-RADS 1), benign (BI-RADS 2) or probably benign (BI-RADS 3 b
b A BI-RADS 3 assessment is coded as negative for diagnostic mammography, but positive for screening mammography.
).Cancer
Defined as a tissue diagnosis of ductal carcinoma in situ or any type of primary invasive breast carcinoma ( Table 2 ). Table 2 also includes a subset of the pathology results that are not included in the definition of cancer for the purposes of the audit.
| Cancer (Breast Cancer) | Not Breast Cancer |
|---|---|
|
|
Coding of Mammograms
True positive
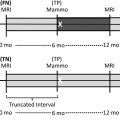
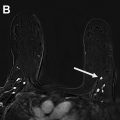
A true-positive result is a tissue diagnosis of cancer within 1 year after a positive examination ( Figs. 1–3 , Tables 3 and 4 ). Remember, a positive screening mammogram is defined differently than a positive diagnostic mammogram ( Box 2 ).


| Biopsy Results | ||
|---|---|---|
| Positive (Tissue Diagnosis of Cancer Within 1 Year) | Negative (Benign Concordant Tissue Diagnosis, or No Tissue Diagnosis of Cancer Within 1 Year) | |
| Positive screening mammogram (BI-RADS 0, 3, 4, 5) | True positive | False positive |
| Negative screening mammogram (BI-RADS 1, 2) | False negative | True negative |
| Biopsy Results | ||
|---|---|---|
| Positive (Tissue Diagnosis of Cancer Within 1 Year) | Negative (Benign Concordant Tissue Diagnosis, or No Tissue Diagnosis of Cancer Within 1 Year) | |
| Positive diagnostic mammogram (BI-RADS 4, 5) a | True positive | False positive |
| Negative diagnostic mammogram (BI-RADS 1, 2, 3) | False negative | True negative |
a Use of BI-RADS 0 at diagnostic mammography is discouraged . An addendum issuing a final assessment should be performed. For the rare instance in which a BI-RADS 0 assessment remains for a diagnostic mammogram, the examination is coded positive.
A woman is recalled from screening for additional imaging (BI-RADS 0). The diagnostic mammogram is suspicious (BI-RADS 4) with a recommendation for tissue sampling. Biopsy yields a breast malignancy. Both the screening and diagnostic examinations are positive and breast cancer is diagnosed within 1 year—therefore, both examinations are classified as true positives.
False positive
A false-positive result is no known tissue diagnosis of breast cancer within 1 year of a positive examination ( Boxes 3 and 4 ). For auditing purposes, 3 separate definitions exist and are outlined in detail in the BI-RADS Atlas.
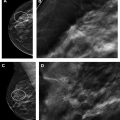
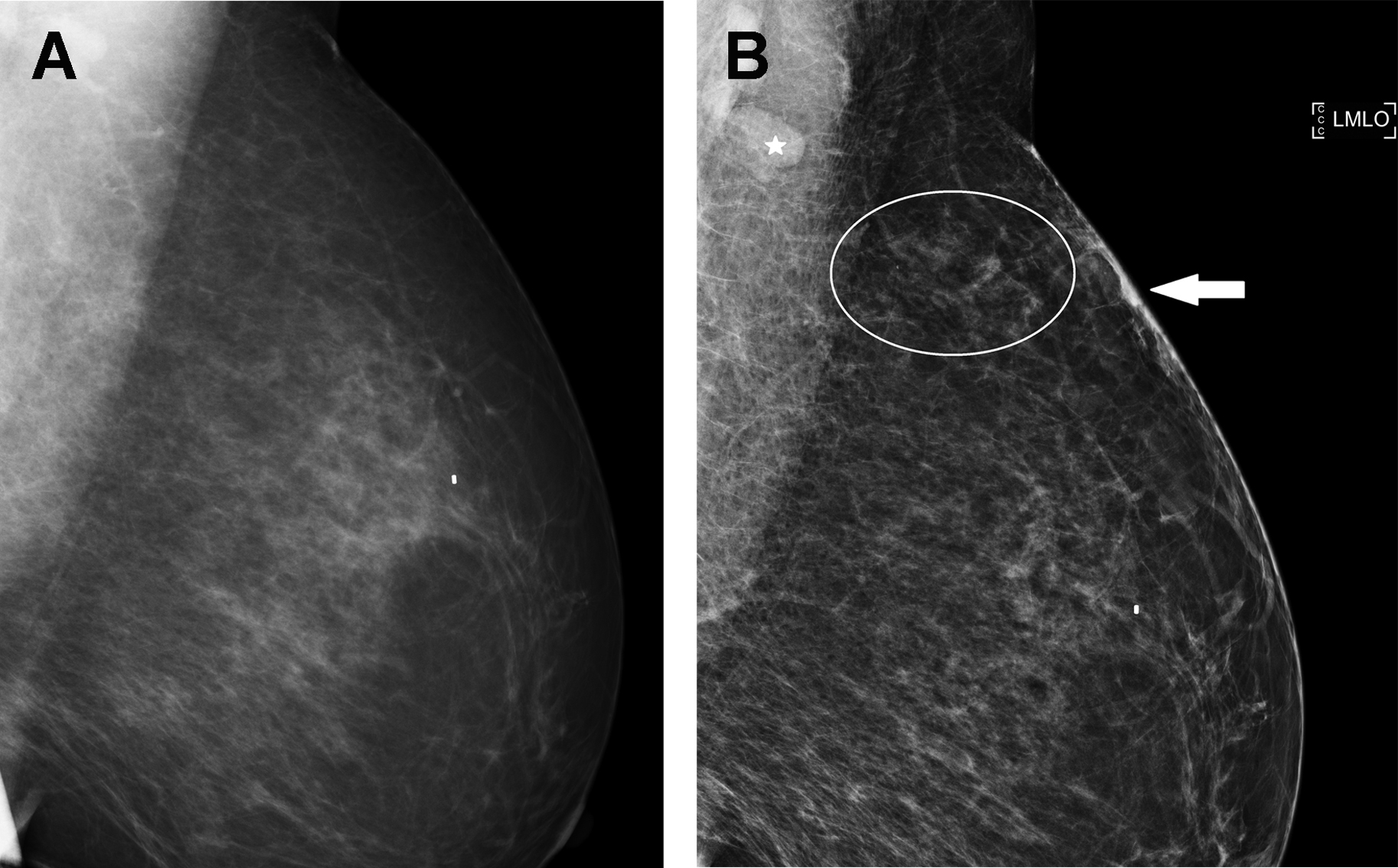
Diagnostic mammography is performed for a new, palpable lump in the left breast (see Fig. 1 ). A suspicious mass is present, and biopsy is recommended (BI-RADS 4). Biopsy yields leiomyosarcoma. The diagnostic mammogram (BI-RADS 4) is classified as false positive because no breast malignancy is diagnosed within 1 year.
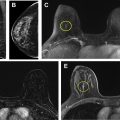
A woman is recalled from baseline screening for additional imaging (BI-RADS 0). On diagnostic mammography, an oval circumscribed mass is assessed BI-RADS 3 (see Fig. 2 ). No breast cancer is diagnosed within 1 year of the examination. The screening mammogram is coded as a false positive, and the diagnostic mammogram is coded as a true negative.
True negative
A true-negative result is defined as no known tissue diagnosis of breast cancer within 1 year of a negative examination (see Box 4 ).
False negative
A false-negative result is defined as a tissue diagnosis of cancer within 1 year of a negative examination ( Box 5 ).
Screening mammography was assigned BI-RADS 2. Patient presents 6 months later with a palpable mass, which is biopsied and yields cancer. The screening examination was interpreted as negative, but malignancy is diagnosed within 1 year, therefore the screening examination is a false negative.
Interval cancer
An interval cancer is a cancer that is diagnosed within 1 year of a negative examination.
Positive Predictive Value
Three separate definitions exist of a positive predictive values (PPV).
- i.
PPV 1 : Abnormal findings at screening ( Box 6 ).
Box 6
PPV1
PPV 1 = True positive/No. of positive screening mammograms
- ii.
PPV 2 : Biopsy recommended. PPV 2 is a metric intended to assess diagnostic mammograms ( Box 7 ).
Box 7
PPV2
PPV 2 = True positive/No. of examinations recommended for tissue diagnosis
- iii.
PPV 3 : Biopsy performed or biopsy yield of malignancy or positive biopsy rate. PPV 3 is a measure designed to assess diagnostic mammograms ( Box 8 ).
Box 8
PPV3
PPV 3 = True positive/No. of biopsies performed
Sensitivity
The sensitivity is the probability of a positive examination when cancer exists ( Box 9 ).
Sensitivity = True positive/(True positive + False negative)
Specificity
The specificity is the probability of a negative examination when cancer does not exist ( Box 10 ). Sensitivity and specificity are only measured with sufficient accuracy if outcomes data are linked to a tumor registry. Therefore, many practices cannot calculate sensitivity and specificity in their audit report.
Specificity = True negative/(True negative + false positive)
Cancer Detection Rate
The cancer detection rate is the number of cancers identified at imaging per 1000 examinations performed. This metric should be calculated separately for screening and diagnostic examinations. In the screening setting, the cancer detection rate should be calculated separately for prevalent cancers (those found at the first round of screening) and for incident cancers (those found at subsequent screening).
Abnormal Interpretation Rate
The abnormal interpretation rate is the percentage of examinations interpreted as positive (see Table 1 ). Calculation of the recall rate adds value only if it represents all positive screening mammograms (BI-RADS 0, 3, 4, or 5) and is therefore equivalent to the abnormal interpretation rate. If screening mammograms are only assessed BI-RADS categories 0, 1, or 2, as recommended by the BI-RADS Atlas, the recall rate and the abnormal interpretation rate will both reflect the percentage of screening mammograms recommended for recall.
The basic clinically relevant mammography audit
The mammography audit allows individuals and practices to measure outcomes and assess if they are meeting the major goals of breast cancer screening: (1) finding a high percentage of cancers that exist in asymptomatic women undergoing screening (measured with cancer detection rate), (2) finding those cancers with an acceptable range of recommendations for additional imaging and biopsies (measured with abnormal interpretation rate and PPV), and (3) finding a high percentage of small, node negative, early stage cancers that are more likely curable (measured with percentage of “minimal,” node-negative, and stage 0 or 1 cancers). ,
Federal regulations specify that a facility’s first audit must be initiated no later than 12 months after certification, and the audit must be completed within an additional 12 months. The additional 12 months are required to collect pathology data and allow time for determination of cancer status. ,
The MQSA requires a facility to have a method to follow-up with all positive mammograms and a system to collect pathology results and to correlate the pathology and mammography results, as well as the mandate to review any known false negatives occurring within 12 months of mammography. However, best practices outlined by the BI-RADS Atlas recommend certain minimum raw data be collected and used to calculate derived data, both of which are steps beyond the federal regulations, to allow for a more clinically relevant measurement of practice and individual outcomes ( Table 5 ).