In this review, several clinical applications of magnetic resonance (MR)-guided focused ultrasound (FUS) are updated. MR-guided FUS is used clinically for thermal ablation of uterine fibroids and bone metastases. Thousands of patients have successfully been treated. Transcranial MR-guided FUS has received CE certification for ablation of deep, central locations in the brain. Thermal ablation of specific parts of the thalamus can result in relief of the symptoms in a number of neurological disorders. Several approaches have been proposed for ablation of prostate and breast cancer and clinical trials should show the potential of MR-guided FUS for these and other applications.
Key points
- •
Thousands of patients with uterine fibroid have successfully been treated with magnetic resonance (MR)-guided focused ultrasound (FUS), leading to technical improvements and increased experience to further improve clinical outcome.
- •
MR-guided FUS has been approved to bring thermal damage to the periosteal nerves, which leads to pain relief from bone metastases and other bone diseases.
- •
Thermal ablation of specific parts of the thalamus with transcranial MR-guided FUS can lead to symptom relief in several neurologic disorders.
- •
MR-guided FUS can be used for more clinical applications (eg, breast and prostate cancer), but clinical trials are needed to prove its potential.
Focused ultrasound
Ultrasound is well known for its application as an imaging modality. To obtain an image, a transducer transmits acoustic waves through the body and receives their reflections at tissue interfaces. Another property of these acoustic waves is that the tissue through which they propagate absorbs their energy. This mechanism is the basis for thermal ablation by focused ultrasound (FUS). By focusing high-intensity acoustic waves, the temperature in the focus increases as a result of energy absorption by the tissue. At a temperature of approximately 56°C for 1 second, irreversible cell death by coagulative necrosis occurs. Furthermore, blood in small vessels can coagulate and stop the blood perfusion. To reach these temperatures, usually an equal amount of ultrasound energy is applied continuously. Because the energy absorption in the ultrasound beam path is lower, the surrounding tissue is spared.
In addition to thermal effects, the tissue can also be damaged through inertial cavitation. Ultrasound waves cause compression and rarefaction of the tissue, and during the latter, gas can be drawn out of solution and bubbles can be created. These microbubbles are compressed and expanded by the ultrasound and can collapse (inertial cavitation), leading to cell damage. Nucleation of such microbubbles can enhance the energy absorption and heating in the focus, a mechanism called enhanced sonication. During an enhanced sonication protocol, short bursts at a very high power are used to form microbubbles in the focus. These microbubbles interact with the acoustic waves, which increases the absorption of energy in the target. Thermal ablation is the primary clinical application for FUS.
Focused ultrasound
Ultrasound is well known for its application as an imaging modality. To obtain an image, a transducer transmits acoustic waves through the body and receives their reflections at tissue interfaces. Another property of these acoustic waves is that the tissue through which they propagate absorbs their energy. This mechanism is the basis for thermal ablation by focused ultrasound (FUS). By focusing high-intensity acoustic waves, the temperature in the focus increases as a result of energy absorption by the tissue. At a temperature of approximately 56°C for 1 second, irreversible cell death by coagulative necrosis occurs. Furthermore, blood in small vessels can coagulate and stop the blood perfusion. To reach these temperatures, usually an equal amount of ultrasound energy is applied continuously. Because the energy absorption in the ultrasound beam path is lower, the surrounding tissue is spared.
In addition to thermal effects, the tissue can also be damaged through inertial cavitation. Ultrasound waves cause compression and rarefaction of the tissue, and during the latter, gas can be drawn out of solution and bubbles can be created. These microbubbles are compressed and expanded by the ultrasound and can collapse (inertial cavitation), leading to cell damage. Nucleation of such microbubbles can enhance the energy absorption and heating in the focus, a mechanism called enhanced sonication. During an enhanced sonication protocol, short bursts at a very high power are used to form microbubbles in the focus. These microbubbles interact with the acoustic waves, which increases the absorption of energy in the target. Thermal ablation is the primary clinical application for FUS.
Magnetic resonance guidance
By performing FUS under image guidance, the target (eg, tumor) can be localized, and the ultrasound focus can be aimed at this target. Two techniques that enable image guidance are ultrasound and MR imaging. The initial FUS treatments were performed under ultrasound guidance, because this technique is inexpensive and has a high temporal resolution. However, the options for treatment planning and monitoring are limited. MR imaging has excellent soft tissue contrast, allowing for three-dimensional treatment planning. Furthermore, real-time temperature information can be obtained, enabling monitoring of thermal damage to ensure coagulative necrosis. After the sonication, MR imaging can be used to assess treatment response, for example, with contrast-enhanced T1-weighted imaging. The contrast agent, gadolinium, does not reach the necrotic tissue, because the blood vessels are damaged. In contrast to well-perfused tissue, no increase in signal intensity on postcontrast T1-weighted images is observed in the necrotic tissue. The percentage of nonperfused volume (NPV) of the target volume can be determined, which can be an indication of the success of the treatment. In this review, the current clinical applications of MR-guided FUS are updated.
Uterine fibroids
Uterine fibroids are common benign tumors in the uterus that can cause abnormal uterine bleeding, pelvic pain, and infertility but are usually symptomless. The ExAblate 2000 (InSightec, Tirat Carmel, Israel) has received both the CE mark (2002) and US Food and Drug Administration (FDA) approval (2004) for the treatment of uterine fibroids. In 2009, Sonalleve (Phillips Healthcare, Vantaa, Finland) received the CE mark. Both FUS systems consist of extracorporeal multielement phased-array transducers, which are built into a special MR table. They operate at a frequency between 0.95 and 1.35 MHz (ExAblate) and 1.2 and 1.5 MHz (Sonalleve) and can be used in combination with 1.5-T and 3.0-T MR scanners.
Since the FDA approval in 2004, thousands of patients have been treated, and several follow-up studies have been performed. The success of treatment has been evaluated based on the volume change of the fibroids, improvement of symptoms, and the reintervention rate. For patients with a higher NPV after the treatment, a lower risk of additional treatment was observed within 1 to 5 years after treatment. The same holds true for older patients, who have a lower risk for reinterventions. Based on pretreatment T2-weighted MR imaging, fibroids can be classified into 3 types: (1) fibroids that appear hypointense, (2) fibroids that are isointense, and (3) fibroids that are hyperintense in relation to skeletal muscle. The NPV of patients with type 3 uterine fibroids was lower than type 1 and 2 fibroids, and these patients required reintervention significantly more often than types 1 and 2. An example of a successful treatment of a type 1 uterine fibroid is shown in Fig. 1 . FUS therapy may not be suitable for type 3 fibroids and MR screening can be used to exclude these patients from FUS treatment. Patients who received neoadjuvant therapy with gonadotropin-releasing hormone agonist, a therapy that decreases the vascularity of the fibroids, had significantly larger NPV at the same applied energy and a lower risk for additional therapy. In a 5-year follow-up study, an overall reintervention rate of 58.6% was reported. When only patients with an NPV larger than 50% were included, the reintervention rate decreased to 50%. Insights and experience from the initial treatments have led to adaptation in patient selection and improvements in the FUS system, with the expectation that long-term outcomes should improve accordingly.
In the initial treatments, long cooling periods between sonications were used to prevent thermal build-up along the ultrasound beam path for multiple overlapping sonications. A new strategy to reduce the cooling period to 22 seconds is the interleave mode, in which the overlap between sonications is minimized by changing the order of the sonications so that the energy absorption in the beam path is decreased. In the new-generation ExAblate system, the transducer can be elevated to minimize the distance from the abdominal wall. This strategy leads to an increase in the maximum energy in the focus and reduces the energy absorption in the near and far field. To limit adverse effects in the beam path, selective transducer elements are automatically disabled if vital structures such as the bowels, bladder, or sciatic nerves are detected in the beam path. In a recent study with 115 patients, these technical improvements, increased experience, and the use of a screening MR imaging examination have improved the NPV to an average of 88%, but follow-up data are not yet available. In this study, patients with hyperintense fibroids on T2-weighted imaging (type 3) were excluded.
An early limitation of MR-guided FUS for fibroids was the long treatment time, often several hours. Both the ExAblate and Sonalleve systems use phased arrays to electronically steer the beam to enlarge the ablated volume during each sonication. The Sonalleve device also has closed-loop feedback, which modulates the power output based on real-time temperature measurements so as to optimize treatment delivery. The technique, along with the interleave mode used with the ExAblate system, has increased the treatment rate and reduced treatment times.
In general, the adverse effects after treatment are minor (eg, transient abdominal pain, mild skin burns, back pain, nausea, and nerve irritation). In a few cases, serious complications were observed: skin burn requiring repair, fibroid expulsion, persistent neuropathy, and abdominal burn. An area that requires more research is the effect of FUS on fertility. Several studies report successful pregnancies after MR-guided FUS treatment, but there has been no study evaluating the effect on fertility of different treatments for uterine fibroids. A clinical trial will compare the safety and effectiveness of MR-guided FUS and uterine artery embolization ( NCT00995878 – clinicaltrials.gov ). Two additional ongoing clinical trials are NCT01142791 – clinicaltrials.gov , which is investigating the use of enhanced sonication to improve clinical outcome, and a multicenter phase 2 and 3 study using the Sonalleve system ( NCT01504308 – clinicaltrials.gov ).
Bone metastases–related pain management
The second MR-guided FUS application that received both CE and FDA approval is bone metastases–related pain management. For this treatment, the ExAblate and Sonalleve (only CE mark) systems can be used. Because cortical bone has high acoustic impedance, a great deal of energy is absorbed by the bone. Therefore, lower energies are applied compared with that used in uterine fibroid treatments. Two mechanisms for FUS-mediated pain relief are suggested. The temperature increase in the cortical bone leads to heating of the periosteal surface, which results in thermal damage to the periosteal nerves, which are responsible for pain perception. The second mechanism is tumor debulking caused by thermal ablation, which diminishes the pressure on the adjacent tissue. The observed pain relief immediately after sonication advocates for the first mechanism, but there is increasing evidence that tumor debulking also plays a role.
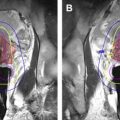
Several hundred patients have been treated who have exhausted, declined, or are unsuitable for other pain palliation methods. The success of the treatment can be evaluated based on changes in the pain scores, quality of life scores, and decrease in pain medication usage. A secondary measure is change in tumor size. In 2 multicenter trials, significant improvements in the pain scores without an increase in the pain medication were observed 3 months after sonication in 64% of 112 and 72% of 25 patients. In 67% of the patients, the dosage of pain medication was decreased. In addition to pain relief, necrosis and increase in the bone density were observed 3 months after FUS treatment. Fig. 2 shows the imaging data of a patient whose treatment led to complete pain relief and reduction in tumor size. There were small areas of NPV seen on postcontrast T1-weighted imaging after the treatment. The use of NPV to evaluate the success of treatment is limited, and more research in the predictive value of this measure is needed. Generally, no or minor adverse events are observed. Observed adverse events included skin burns, sonication pain, fractures, neuropathy, posttreatment fatigue, and skin numbness.
MR-guided FUS also shows promise for pain relief in other bone diseases. In 2 studies, patients with osteoid osteoma were treated and in 90% to 100% of the patients symptoms were completely resolved at a 6-month to 12-month follow-up. Eighteen patients with facet joint osteoarthritis were successfully treated, and a decrease in pain was observed, together with an improvement in disability. Pain relief from osteoarthritis was also observed in 6 of 8 patients who were treated with MR-guided FUS for medial knee pain. No adverse events were observed in any of these studies, supporting the use of MR-guided FUS for bone pain management in a range of bone diseases.
Brain disease
FUS has great potential for treating brain disease, because the technique could be used to ablate targeted tissue without injuring the normal brain. In contrast, during conventional neurosurgery, damage to normal brain tissue is usually inevitable, especially when deep-seated brain structures are involved. The challenge for FUS in the brain is the high acoustical impedance of the skull. The high impedance leads to absorption of a great part of the applied energy, resulting in heating of the skull. This situation may, in turn, increase the temperature in the brain tissue adjacent to the skull. To minimize skull heating, a hemispherical design for the transducer is chosen, so that the applied energy is distributed over a larger area. A lower operating frequency reduces absorption in the bone, at the cost of an increase focal size. It was determined that a frequency of approximately 700 kHz is optimal for transcranial FUS. Another issue concerning the acoustic impedance of the skull is the large difference between the impedance of the skull and that of brain tissue. This difference leads to refraction of the acoustic waves and distortion of the focus. The focus can be restored by using many transducer elements, each with an optimized phase. The required phase corrections can be calculated from radiograph-computed tomography, from which the spatial distribution of the skull thickness and density can be determined. Thermal ablation using FUS in the brain should be performed under MR guidance, because high-resolution anatomic images are a prerequisite for proper treatment planning. The ExAblate Neuro system (InSightec) consists of a hemispherical 1024-element phased-array transducer, which operates at 650 kHz. This device has received CE mark for targets in the thalamus, subthalamus, and pallidum.
A precursor of this system (ExAblate 3000, 512 elements, 670 kHz) was used to show, for the first time, the ability to focus therapeutic ultrasound through the skull into the brain ( Fig. 3 ). In 3 male patients with glioblastoma, with tumors located deep and central in the brain, it was shown that the target tissue can be heated, and significant heating in the tissue close to the skull was prevented. Because the researchers were limited by the power of the device (650–800 W), it was estimated that they did not achieve coagulative necrosis. Extrapolation of their results suggests that this treatment should be possible without overheating the tissue at the brain surface. However, the targetable regions may be limited to deep, central locations in the brain. This factor makes transcranial MR-guided FUS particularly suitable for treating neurologic disorders such as essential tremor, Parkinson disease, and neuropathic pain. Ablation of specific parts of the thalamus can result in relief of the symptoms in these diseases.