Abstract
In the imaging evaluation of hearing loss, the radiologic examination is complementary to the physical examination. The clinical evaluation includes not only an accurate history of the hearing loss itself (unilateral or bilateral, slow onset or fast onset, etc.) but also the patient’s medical history (possible trauma, ototoxic drugs, associated facial nerve palsies, etc.). Hearing loss can be described as sensorineural, conductive, or mixed, and this will guide the choice of imaging modality to correlate with the clinical history and symptoms. In this chapter, we will review the more common hearing loss entities and divide these pathologies into those presenting with sensorineural hearing loss (SNHL), conductive hearing loss (CHL), and mixed hearing loss (MHL). The pathologies for SNHL and CHL will be further grouped and discussed as either congenital or acquired entities. In very simplistic terms, CHL is frequently initially evaluated with CT, whereas SNHL is most frequently initially evaluated with MRI. When reviewing the imaging studies, a systematic “outside-in” approach along the hearing pathway is helpful, from the external anatomic structures to the internal structures.
Keywords
Computed tomography (CT), Conductive hearing loss (CHL), Hearing loss (HL), Magnetic resonance imaging (MRI), Mixed hearing loss (MHL), Sensorineural hearing loss (SNHL), Temporal bone (TB)
Disclosure
Disclosure of any relationship with a commercial company that has a direct financial interest in subject matter or materials discussed in article or with a company making a competing product.
Introduction
In the imaging evaluation of hearing loss, the radiologic examination is complementary to the physical examination. The clinical evaluation includes not only an accurate history of the hearing loss itself (unilateral or bilateral, slow onset or fast onset, etc.) but also the patient’s medical history (possible trauma, ototoxic drugs, associated facial nerve palsies, etc.). Hearing loss can be described as sensorineural, conductive, or mixed, and this will guide the choice of imaging modality to correlate with the clinical history and symptoms. In this chapter, we will review the more common hearing loss entities, and divide these pathologies into those presenting with sensorineural hearing loss (SNHL), conductive hearing loss (CHL), and mixed hearing loss (MHL). The pathologies for SNHL and CHL will be further grouped and discussed as either congenital or acquired entities. In very simplistic terms, CHL is frequently initially evaluated with CT, whereas SNHL is most frequently initially evaluated with MRI. When reviewing the imaging studies, a systematic “outside-in” approach along the hearing pathway is helpful, from the external anatomic structures to the internal structures.
Imaging Modalities
Thin-section CT and MRI provide complementary information in evaluating skull base and temporal bone (TB) pathologies. On evaluation of a patient with hearing loss, CT of the TB is particularly helpful to evaluate the osseous structures (external auditory canal [EAC], middle ear, and otic capsule), whereas MRI is useful to evaluate the inner ear structures, internal auditory canal (IAC), cerebellopontine angle (CPA), and brainstem.
CT of the TBs can be performed with the patient lying supine, in the anatomic axial plane. Multidetector CT scanners acquire submillimeter slices with low radiation dose and reduced scan time. It is important for the imager to be wary of low-dose techniques, as these will limit the accurate evaluation of the normally dense TB osseous structures. Submillimeter multiplanar reconstructions can be performed with minimal overlap. Although parameters vary by institution, suggested TB CT parameters are described in Box 8.1 . This technique is capable of revealing a broad spectrum of EAC and middle ear lesions that may not be apparent on the basis of clinical findings alone. Radiation dose in TB CT imaging can be high because of the requirement of high spatial resolution. Leng et al. demonstrated an ultrahigh-resolution scan mode with an iterative reconstruction algorithm to improve the quality of the TB CT image with decreased radiation dose. Axial acquisitions with multiple plane reconstructions can effectively evaluate the osseous TB anatomy and pathology ( Fig. 8.1 ).
Submillimeter (0.6 mm) slices
Multiplanar reconstructions with minimal overlap (0.5 mm)
Axial
Coronal
Long-axis (Stenver) projection
Short-axis (Pöschl) projection
Scout image
Standard algorithm large field of view thicker sections (2–3 mm)
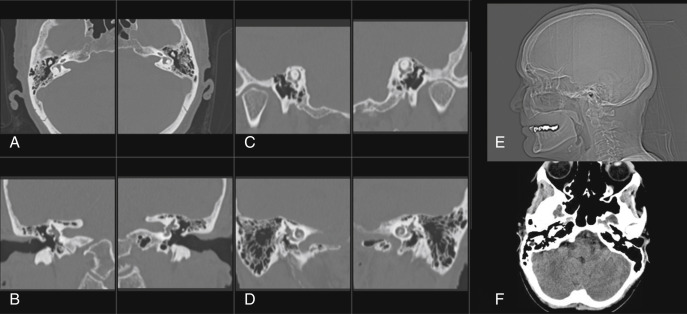
MRI is a fundamental method of evaluating the inner ear structures as well as the cerebrospinal fluid (CSF) spaces, IAC, CPA, and adjacent brain parenchyma. Dedicated head and temporomandibular joint coils are important for achieving an optimal signal to noise ratio. Screening MRI (noncontrasted thin-section CSF bright sequences) of the CPA/IAC can be performed in the initial evaluation of SNHL in the appropriately screened patient (i.e., older patient with progressive asymmetric hearing loss). When there is a suspected or known retrocochlear lesion, MRI of the CPA/IAC without and with contrast can be performed. In addition, in cases of complicated SNHL, or when there are other symptoms such as cranial neuropathy, long tract signs, and/or headache, imaging should include whole brain and posterior fossa sequences (axial T2 and/or fluid-attenuated inversion recovery [FLAIR]). Although parameters vary by institution, suggested screening MRI IAC parameters are described in Box 8.2 . Imaging is critical in the assessment of hearing loss, and when a pathology is identified, the most commonly encountered tumor is vestibular schwannoma (VS). One diagnostic review and meta-analysis concluded that nonimaging screening protocols, including pure-tone audiometry, auditory brainstem response, clinical symptoms, electronystagmography, caloric irrigation, and hyperventilation tests, were not accurate in detecting VSs.
Axial and coronal thin-section T1
Axial T2 SPACE
Axial diffusion-weighted imaging
Axial and coronal T1 with contrast and fat saturation
SPACE , sampling perfection with application optimized contrast using different flip angle evolutions.
MRI is widely used for the evaluation of asymmetric SNHL. There are, however, drawbacks of risks, cost, and time associated with MRI. Non-contrast-enhanced MRI studies have been shown to be adequate in the detection of CPA/IAC masses using thin CSF bright sequences. A prospective, blinded study using fast spin echo (FSE) T2-weighted (T2W) MRI demonstrated a threefold decreased cost while maintaining 98% sensitivity for schwannomas. A screening high-resolution FSE T2W MRI was also shown to be adequate to detect other pathologies, including other CPA/IAC lesions, inner ear lesions, and intraaxial lesions (such as infarctions, multiple sclerosis, mesial temporal sclerosis, and colloid cysts). Daniels et al. concluded that this high-resolution FSE screening technique, used in conjunction with appropriate clinical prescreening and referral, can provide an equally sensitive method of evaluating unilateral SNHL compared with contrast-enhanced T1-weighted (T1W) MRI while reducing costs and providing distinct advantages in evaluating non-VS causes of SNHL. They also showed that false positives on contrast-enhanced T1W MRI (true negatives on FSE T2W MRI) can lead to unnecessary diagnostic tests, intervention, and increased costs of care. Similarly, in a study comparing T2W MRI with contrast-enhanced MRI in 146 patients with asymmetric SHNL, Verret et al. demonstrated that only T2W MRI instead of contrast-enhanced MRI would have decreased costs over $100,000. With the addition of multiple planes (i.e., axial and coronal) and advances in MRI techniques (e.g., constructive interference in steady state [CISS], fast imaging employing steady-state acquisition [FIESTA], and sampling perfection with application optimized contrast using different flip angle evolutions [SPACE]), 100% sensitivity and excellent interrater agreement can be achieved with high-resolution MRI protocols.
Contrast-enhanced MRI has been shown by other studies to provide useful information for nonneoplastic lesions. Annesley-Williams et al. found that 2- to 5-mm lesions may be missed on two-dimensional (2D) FSE T2W MRI and that contrast-enhanced MRI may be needed to further investigate findings on three-dimensional (3D) and 2D MRI (9% and 15%, respectively). However, in considering patient care related to nonneoplastic CPA/IAC pathologies, such as inflammatory pathologies that are typically acute in onset and of viral etiology, these cases are treated regardless of the MRI findings.
Imaging pitfalls should also be considered when evaluating MRI. For example, the petrous apex marrow adjacent to the IAC can enhance on postcontrast T1W MRI (especially bright on cases without fat saturation) and be erroneously interpreted as a CPA/IAC tumor. Similarly, asymmetric aeration of the petrous apices with unilateral benign trapped fluid can also be mistaken for pathology. The enhancement of a prominent anterior inferior cerebellar artery (AICA) loop may be mistaken for a small VS. Other pseudomasses include the choroid plexus protruding through the lateral recesses of the fourth ventricle and the cerebellar flocculus normally projecting into the posterolateral aspect of the CPA cistern. The vestibular nerve ganglion (Scarpa ganglion) in the IAC should also not be mistaken as a small VS. If there is a punctate (<2 mm) enhancing lesion in the CPA/IAC, it can safely be followed over time. Pathologies arising from the jugular foramen may also extend superiorly into the CPA/IAC region.
Sensorineural Hearing Loss
SNHL indicates dysfunction of the cochlea, cochlear nerve, and/or brain and can be measured with a tuning fork touched to the top of the head, by conduction through bone, so that the sound will localize to the normal side in these patients. The structures of note include the cochlea and the cochlear nerve from the origin nucleus through the CPA/IAC cistern into the modiolus. In addition to CT of the TBs, thin-section MRI with T1W pre- and postcontrast with fat-saturation sequences, T2W sequences, and 3D CISS/3D T2 FSE/3D fourier transformation CISS/SPACE sequences are essential for the thorough evaluation of SNHL.
Congenital SNHL Pathologies
Congenital hearing loss is defined as hearing loss present at birth and is generally divided into genetic and nongenetic forms. Congenital SNHL etiologies include membranous labyrinth dysplasias, cochlear/vestibular aplasia/dysplasia, enlarged vestibular aqueduct, IAC atresia/stenosis, and perilymphatic fistula. Genetic anomalies cause approximately half of the cases of congenital SNHL ; of these approximately 75%–80% demonstrate an autosomal recessive inheritance, 15%–20% demonstrate an autosomal dominant inheritance, and 1%–2% demonstrate an X-linked inheritance. Approximately 30% of inherited forms of hearing loss are syndromic, and the remaining 70% are considered nonsyndromic.
Although the cause is unknown in 25%–40% of cases, intrauterine toxin exposure, infections, and perinatal insults account for many cases of pediatric SNHL. Cytomegalovirus (CMV) infection is the most common environmental cause of prelingual hearing loss in the United States, implicated in approximately 10% of infants with congenital hearing loss and 34% of children with moderate to severe late-onset idiopathic hearing loss. Among children with clinically apparent congenital CMV infection, the prevalence of SNHL is ∼30%.
Cross-sectional imaging is an integral tool in the clinical evaluation of congenital SNHL. Otolaryngologists utilize CT and/or MRI to identify potential etiologies and abnormalities that may predict hearing loss progression or prognosis, to define TB anatomy and the central auditory pathway and to identify additional intracranial abnormalities that may require further workup and/or intervention. Abnormalities on CT and/or MRI are found in 20%–50% of children with SNHL and correlate with the degree of hearing loss. Although traditionally bone algorithm CT is the modality of choice in demonstrating inner ear dysplasias in children with SNHL, dual-technique imaging with high-resolution TB CT and MRI has been show to identify a substantially larger number of abnormalities in children being evaluated for cochlear implantation than either technique alone.
The spectrum of inner ear anomalies ranges from complete aplasia to subtle dysplasia. Sennaroglu and Saatci proposed a classification for cochleovestibular malformations that included, in order of decreasing severity: labyrinthine aplasia, cochlear aplasia, common cavity deformities, cystic cochleovestibular malformations (incomplete partition type-1), cochleovestibular hypoplasia, and incomplete partition type-2 (IP-II), each of which is thought to result from an insult occurring at a progressively later stage of development.
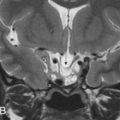
Labyrinthine aplasia (Michel deformity) is the complete absence of the cochlea, vestibule, and semicircular canals (SCCs). In cases of labyrinthine aplasia, there is SNHL from birth without the option of cochlear implantation in the affected ear. The bony labyrinth is featureless, and the petrous apex may be hypoplastic or absent, and the IAC is small. The middle ear may be normal in the mild form to abnormal with fused ossicles in the more severe form. In these cases, the lateral wall of the inner ear is flat and the facial nerve geniculate ganglion is displaced posterior to its normal expected location. There is no normal high-signal-intensity fluid in the membranous labyrinth on high-resolution T2W MR. Labyrinthine aplasia occurs when there is arrest of the otic placode development at the third gestational week. It may be associated with Klippel-Feil syndrome and/or thalidomide exposure ( Fig. 8.2 ).
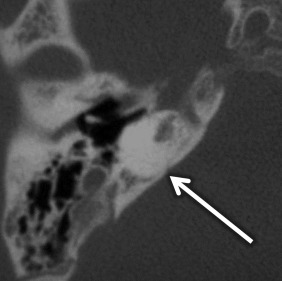
Cochlear aplasia occurs in late third week arrest, with absent cochlea, dysmorphic vestibule, and SCCs. The vestibule and IAC may be dilated. In these cases, the cochlear promontory is flat and the facial nerve labyrinthine segment, geniculate ganglion, and anterior tympanic portions are located at the expected location of the cochlea. The EAC, middle ear, ossicular chain, bony vestibular aqueduct, and endolymphatic duct are normal in size ( Fig. 8.3 ).
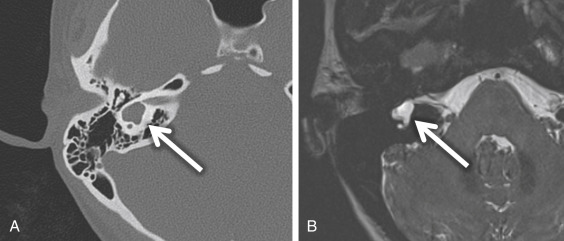
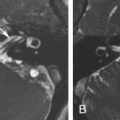
If there is an arrest in the fourth week after differentiation of the otic placode into the otocyst, a common cavity deformity occurs and an ovoid single inner ear cavity is formed. The SCCs are usually absent but may be present and dysplastic. The IAC size is concordant with the size of the common cavity and enters the cavity at the center portion; however, the modiolus is absent ( Fig. 8.4 ). Oblique sagittal T2W images perpendicular to the IAC are important to assess the presence or absence of the cochlear nerve if cochlear implantation is planned ( Fig. 8.5 ).
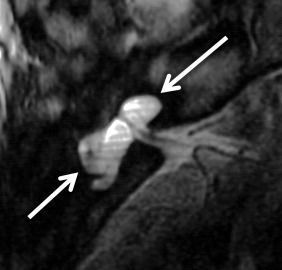
A cystic cochleovestibular anomaly results if there is arrest in the fifth gestational week. A “figure 8”-shaped dysplastic and cystic cochlea and vestibule with SCC dysplasia is seen. There are no internal features, and no modiolus is identified in these cases. The vestibule is cystic and dilated, and the SCCs are variable in shape and degree of dilatation. The IAC is normal or small in size with a normal facial nerve; however, the cochleovestibular nerves are deficient or absent. The EAC, middle ear, ossicular chain, bony vestibular aqueduct, and endolymphatic duct are normal in size.
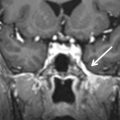
SCC dysplasia is a spectrum of congenital deformities. The embryologic insult occurs at 6–8 weeks of gestation. The SCCs begin as disk-shaped evaginations arising from the vestibular appendage in the sixth gestational week. The central portion of each disk is resorbed and replaced by the mesenchyme, which results in the formation of the characteristic SCC. Failure of formation of one of these disks results in the absence of the involved SCC, whereas incomplete absorption of the central portion of the disk results in a dysplastic or pocket-shaped SCC. The superior SCC is the first to form, followed by the posterior SCC and then the lateral SCC. The most common and least severe dysplasia in this spectrum is a common cavity formed by the lateral SCC and the vestibule ( Fig. 8.6 ). On axial CT images, subtle SCC abnormalities may be indicated by an absent or small bony island between the vestibule and the lateral SCC ( Fig. 8.7 ). Normally, the transverse diameter of this bony island measures between 2.6 and 4.8 mm. As an internal standard for comparison, the osseous bony island between the vestibule and inside the loop of the lateral SCC should be wider from right to left than the vestibule width from right to left. In these cases, there may be associated oval window atresia (OWA) and the cochlea may be affected with incomplete partition of the apical and middle turns. There may also be ossicular anomalies. In addition to SNHL, there may be CHL due to this OWA and ossicular chain anomalies. It can be sporadic or associated with congenital syndromes (e.g., CHARGE, Alagille, Waardenburg, Crouzon, Apert). In CHARGE syndrome (coloboma, heart defects, choanal atresia, retardation of growth and development, and ear abnormalities), for instance, there is absence of the SCC bilaterally, dysmorphic vestibule, and cochlear anomalies with absence of the cochlear nerve canal.
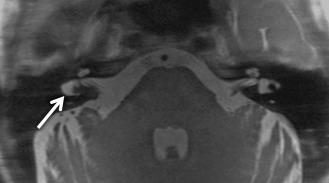
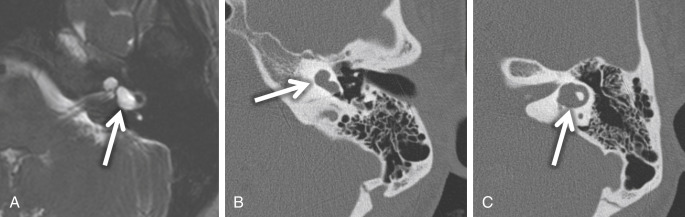
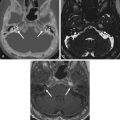
Large endolymphatic sac anomaly (often used when found on MRI), or large vestibular aqueduct syndrome (often used when found on CT), is seen when there is arrest in the seventh gestational week ( Fig. 8.8 ). This is the most common inner ear malformation seen in children with nonsyndromic hearing loss and is bilateral in 90% of cases. The prevalence of enlarged vestibular aqueducts in children with SNHL is estimated to be 10%–15%. There are often associated other inner ear anomalies, such as IP-II, vestibular enlargement, and SCC dysplasias. It is a familial lesion with autosomal recessive inheritance. Patients are able to hear at birth, but their hearing deteriorates over the early years of life. The typical clinical presentation is a child or teenager with progressive SNHL. There may be posttraumatic potentiation of SNHL. It is the most commonly missed cause of congenital deafness. Rarely, patients have distal renal tubular acidosis resulting in varying degrees of metabolic acidosis. There is also an association with Pendred syndrome, which is severe SNHL with thyroid pathology.
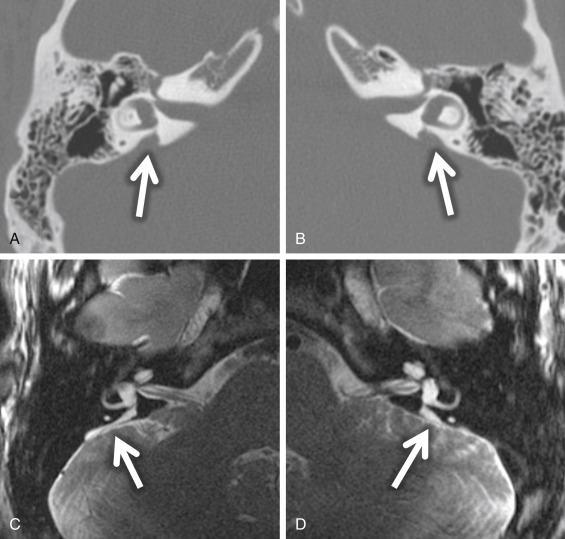
The criteria to determine an enlarged vestibular aqueduct (in which the endolymphatic sac lies) are variable. On CT, a bony vestibular aqueduct diameter measuring greater than 1.5 mm in the transverse dimension at the midpoint or an opercular measurement greater than 2 mm is generally considered to be the defining characteristics. The vestibular aqueduct is usually found at the level of the vestibule and lateral SCC, so it is easy to simply compare the diameter of the duct/sac with the lateral and posterior SCC width, which should be larger than the aqueduct ( Fig. 8.8A and B ). There is no relationship between the size of the endolymphatic sac and the severity of the SNHL. The aqueduct itself is best evaluated with CT, whereas the sac is best seen as bright signal intensity on thin-section T2W MRI. In greater than 75% of cases, there is an associated cochlear dysplasia. The apical turn of the cochlea in these cases is dysmorphic with modiolar deficiency ( Fig. 8.9 ). High-resolution T2W MRI may be able to distinguish more subtle abnormalities of scalar chamber asymmetry with the more anterior scala vestibuli larger than the more posterior scala tympani. Approximately 50% of cases have associated vestibular and/or SCC anomalies. In patients with sudden hearing loss, studies have shown wider vestibular aqueducts in the affected ear as compared with controls. The endolymphatic sac can show enhancement, which may be due to inflammation of the endolymphatic tissue or venous engorgement. It is hypothesized that a wide vestibular aqueduct may be associated with insufficient maturation of the inner ear. The congenital “fragile” inner ear may receive abnormal pressure transmission through the vestibular aqueduct.
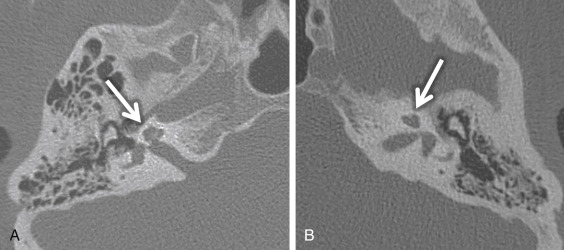
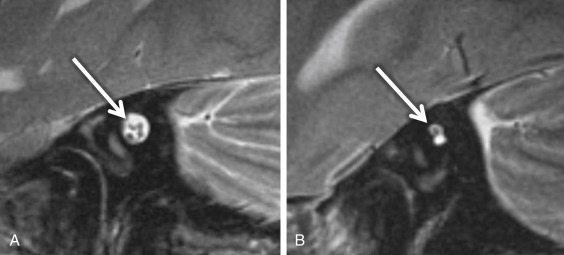
Cochlear nerve deficiency (CND) is noted in 12%–18% of pediatric patient ears with SNHL. It is usually congenital and refers to the absence or reduction in caliber of the cochlear nerve. Because cochlear implants are generally contraindicated in CND, it is important to identify this condition in children being considered for implantation. There will be an associated narrowing of the IAC on CT (IAC diameter of <4 mm), which is theorized to occur because the IAC width depends on the presence of the vestibulocochlear nerve cells to form normally. In general, pediatric patients with narrow IACs on CT perform worse after implantation than those with normal-caliber IACs, presumably because the cochlear nerve is likely to be absent or small when the IAC is narrow. With CND, CT may also show a stenotic or absent bony cochlear canal (normally between 1.4 and 3.0 mm). The osseous anatomy must be assessed with caution, as cochlear nerve-deficient ears may demonstrate normal caliber of the bony cochlear canal in ≤23% of cases and normal-sized IACs in ≤73% of cases. Heavily T2W or CSF bright MRI sequences, such as SPACE, CISS, and FIESTA, can be used to identify the cochlear nerve, which should be located in the anterior inferior quadrant of the IAC, as well as the intracanalicular segment of the facial nerve (anterior and superior) and the superior and inferior divisions of the vestibular nerve posteriorly within the IAC ( Fig. 8.5A ). Although CND can be seen in isolation, it can occur in conjunction with aplasia of the vestibular nerve (complete absence of the eighth cranial nerve) ( Fig. 8.5B ).
Rarely, it has been reported that an intrameatal AICA loop can cause asymmetric SNHL because of neurovascular compression of the cochlear nerve. The study by Gorrie et al. found a statistically significant association between AICA loops that ran between the facial and vestibulocochlear nerves and hearing loss but found no statistically significant association between AICA loops that made no contact with the nerve, ran adjacent to the nerve, or displaced the nerve.
Acquired SNHL Pathologies
Acquired SNHL etiologies include tumors, infectious/inflammatory processes, trauma, autoimmune/immune disorders, and degenerative/idiopathic processes. Tumors can cause SNHL, including vestibular (acoustic) schwannomas (60%–90%), meningiomas (3%–6%), epidermoids (3%–6%), schwannomas of cranial nerves 5, 7, 9, 10, 11, and 12, lymphoma, leukemia, metastases, and meningeal carcinomatosis.
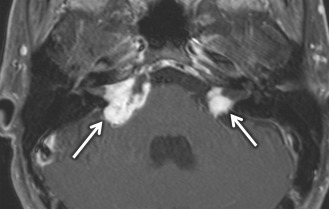
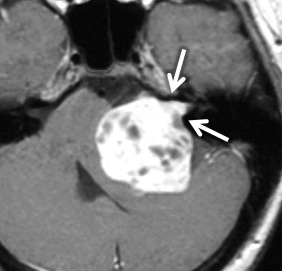
VS is the most common tumor of the IAC/CPA, accounting for 60%–90% of tumors in this region. In sporadic cases, the incidence is highest in patients in the fifth through the seventh decades. However, in patients with neurofibromatosis type II, patients commonly present in the first 2 decades with bilateral VSs ( Fig. 8.10 ) along with other cranial nerve schwannomas, meningiomas, and ependymomas of the central nervous system. In addition to SNHL, patients can have symptoms of tinnitus, dysequilibrium, and/or decreased speech discrimination, because of the mass effect of the tumor on the cochlear and vestibular divisions of cranial nerve 7.
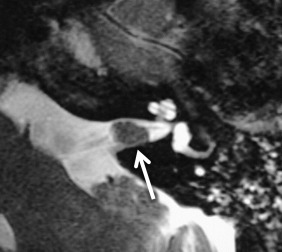
On CT, VSs are isoattenuating compared with the brain parenchyma and can be difficult to delineate without contrast material enhancement. Lateral extension of the VS from the CPA into the IAC results in widening of the porus acusticus ( Fig. 8.11 ). The spherical cisternal component forms an acute angle with the petrous bone. Hemorrhage and calcification are rare in untreated tumors. Enhancement is typically avid and homogenous and better seen on MRI. On T1W MRI, VSs are usually isointense or mildly hypointense compared with brain parenchyma and hyperintense compared with CSF. On T2W MRI, they are mildly hyperintense compared with brain parenchyma and isointense to hypointense compared with CSF. If large, the tumors may have a heterogeneous internal architecture with cystic components ( Fig. 8.11 ). Deformity and mass effect on the adjacent structures can result in facial numbness if the trigeminal nerve is compressed, cerebellar signs if the lower cranial nerves are affected, or hydrocephalus if there is compression of the fourth ventricle. Heavily T2W or CSF bright MRI sequences can outline the tumor relative to adjacent structures ( Fig. 8.12 ). There may be decreased labyrinthine signal intensity, which may be due to increased protein content.
The VSs are slow-growing lesions, with growth rates ranging from 0.2 mm to a few millimeters per year. Surgical resection for complete cure is performed, if feasible. Of the three main surgical approaches (retrosigmoid, middle cranial fossa, and translabyrinthine), the translabyrinthine approach is reserved for patients with poor hearing or complete hearing loss, because it leads it to hearing sacrifice. When the VS is “impacted,” extending laterally into the cochlear aperture, there is a decreased chance of hearing preservation after surgery. Stereotactic radiosurgery is an option in high-risk patients, those with bilateral tumors, and those with residual tumors after initial treatment.
The VSs can also arise from Schwann cells within the membranous labyrinth, described as an intralabyrinthine schwannoma (you may have noticed on the right if you were looking closely at Fig. 8.6 ). This benign tumor appears on MRI as a soft tissue density lesion within the high-signal inner ear fluid and shows focal enhancement. It appears as a focal enhancing mass within the membranous labyrinth on contrast-enhanced T1WI MRI and as a filling defect on high-resolution T2W MRI. These can be subdivided according to location within the inner ear structures: intracochlear, vestibulocochlear, transmodiolar, transmacular, and transotic. Intralabyrinthine schwannomas result in progressive hearing loss.
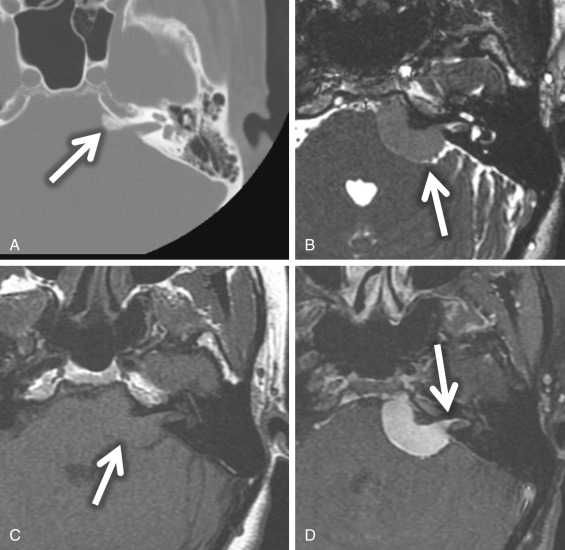
Meningiomas are also found in the CPA but are most commonly found eccentric to the medial opening of the IAC, the porus acusticus ( Fig. 8.13 ). Possible IAC extension of a meningioma usually does not result in widening of the porus acusticus as it does with VSs. These meningiomas are characteristically broad based along the petrous wall with a hemispheric morphology and form an obtuse angle at the bone-tumor interface. Meningiomas are hyperattenuating or isoattenuating compared with brain parenchyma and may be calcified on CT. A helpful imaging feature is the adjacent bone, which may be sclerotic or hyperostotic ( Fig. 8.13A ). On MRI, meningiomas are isointense or slightly hypointense compared with gray matter on T1W MRI and isointense or hypointense compared with gray matter on T2W MRI. Typically, meningiomas show homogeneous enhancement unless there is internal calcification or microcystic change. A “dural tail” of dural enhancement extending from the margins of the lesion is often seen and may extend into the IAC itself ( Fig. 8.13D ). Surface CSF cleft and flow voids from marginal pial vessels may be seen, often described as a CSF/vascular cleft between the extraaxial meningioma and the adjacent normal brain parenchyma ( Fig. 8.13B ).
An endolymphatic sac tumor (ELST) is a locally invasive papillary cystadenomatous tumor of the endolymphatic sac in a retrolabyrinthine location. These lesions are centered in the fovea of the endolymphatic sac along the posterior surface of the petrous TB. Larger lesions (greater than 3 cm) can spread to involve the middle ear, CPA cistern, and/or jugular foramen. On CT, this lesion shows intratumoral bone spicules with a moth-eaten, lytic appearance of the invaded bone. A thin rim of calcification along the posterior margin of the tumor is common ( Fig. 8.14A ). A vascular catheter or CT angiogram may exhibit enlarged distal vessels from the ascending pharyngeal and occipital arteries feeding the vascular tumor. Flow voids can be seen when the tumor is greater than 2 cm in size. On T1W MRI, lesions greater than 3 cm and along the margin when the lesion is less than 3 cm may show high-signal foci, reflecting blood products ( Fig. 8.14B ). The majority of ELSTs (80%) have these foci of increased signal intensity. Low-intensity foci in the tumor matrix may represent hemosiderin staining. These lesions show heterogeneous enhancement ( Fig. 8.14C ).
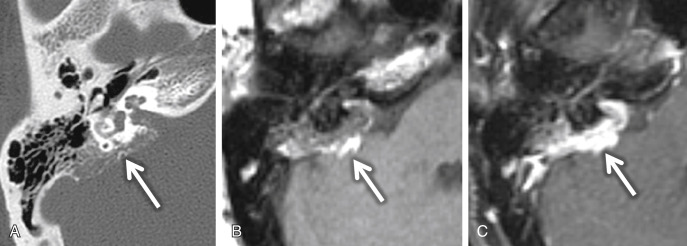
Virtually all patients with ELSTs will have SNHL. Other symptoms include facial nerve palsy, pulsatile tinnitus, and vertigo. Hearing loss and ELST are frequently associated with von Hippel-Lindau syndrome (VHL) and should be considered when screening individuals at risk for VHL and when monitoring patients with an established diagnosis of VHL. Although most cases are sporadic, ∼7% ELSTs may be seen in the setting of VHL. Bilateral ELSTs are seen in VHL. Audiologic evaluation and MRI should allow early detection and enhance management of hearing loss in these patients. Many patients with VHL have hearing loss without radiographic evidence of an ELST. Whether it is caused by an ELST that is too small to be detected by MRI or is produced by some other etiology is still unknown.
Labyrinthine ossificans (LO) is also a potential cause of acquired SNHL ( Fig. 8.15 ). There are three stages to labyrinthitis (vestibulocochlear neuritis). In the acute stage, MRI may show inner ear enhancement. In the intermediate stage, fibrous tissue results in loss of the fluid signal on heavily T2W MRI (e.g., SPACE, CISS, or FIESTA) ( Fig. 8.15C and D ). However, there is higher T2 signal than as seen with an intralabyrinthine schwannoma. CT may appear normal in these early stages. In the late stage, the inner ear structures show bone attenuation on CT ( Fig. 8.15A and B ). The most extensive cases are seen as a complication of meningitis. TB trauma (e.g., a unilateral fracture with a perilymphatic fistula or postoperatively after stapedectomy or other inner ear surgery) and autoimmune inner ear disease have also been attributed to the development of LO. The scala tympani of the basal turn is the most common region of cochlear ossification. Patients present acutely with profound SNHL, vertigo, and tinnitus. LO can complicate or may preclude cochlear implantation.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree