Nigel C. Cowan, Richard H. Cohan
Urothelial Cell Cancer, Upper Tract and Lower Tract
Upper Tract Urothelial Carcinoma
Definition
Upper tract urothelial carcinoma (UTUC) refers to malignant changes of the uroepithelial cells lining the urinary tract anywhere from the renal calyces to the ureteral orifice.
Epidemiology
Urothelial carcinomas are the fourth most common tumours, after prostate or breast cancer, lung cancer and colorectal cancer.1–3 They may be located in the lower urinary tract; the bladder or urethra, or the upper urinary tract; renal calyces, renal pelvis and ureter. Bladder cancers (BCa) account for 90–95% of urothelial carcinomas and are the second most common malignancy of the urogenital tract after prostate cancer.2 UTUCs are uncommon and account for only 5–10% of urothelial carcinomas. Urothelial carcinomas account for 90% of tumours of the renal pelvis; other epithelial cell types include squamous cell carcinoma, adenocarcinoma, carcinosarcoma, small cell carcinoma and papilloma.
The accuracy of incidence and mortality statistics for renal pelvic UTUC in the USA is limited by the fact that UTUC and renal cell carcinoma are grouped together for reporting purposes.1 The estimated annual incidence of UTUCs in Western countries is approximately 1–2 new cases per 100,000 inhabitants. Pyelocalyceal tumours are about as twice as common as ureteral tumours. In 8–13% of cases a concurrent bladder cancer is present. Recurrence of disease in the bladder occurs in 30–51% of UTUC patients4 and recurrences in the contralateral upper tract are observed in 6%.5
The natural history of UTUCs is different from that of bladder cancer; 60% of UTUCs are invasive at diagnosis compared with only 15% of bladder tumours.
Hereditary cases of UTUC are linked to hereditary non-polyposis colorectal carcinoma (HNPCC), which should be suspected if the patient is <60 years of age or has a history of an HNPCC-type cancer.6–8
Risk Factors
UTUC has a peak incidence in patients in their 70s and 80s. UTUC is three times more prevalent in men than in women predominantly because of the higher rates of smoking and occupational exposure to carcinogens among men.9
Chemical carcinogens predispose the entire urothelium to the development of multifocal urothelial carcinoma10 by an effect sometimes referred to as field change of the urothelium. Smoking is the principal risk factor for UTUC.11 Occupational exposure is most commonly caused by aromatic amines found in certain chemicals used in many industries, e.g. dyes, textiles, rubber, chemicals, petrochemical and coal and responsible for ‘amino tumours’. Chemicals such as benzidine and β-naphthalene were banned in the 1960s in most industrialised countries. Usually a UTUC caused by occupational exposure to aromatic amines is also associated with a bladder tumour. The average duration of exposure needed for a UTUC to develop is 7 years.10
UTUCs resulting from the drug phenacetin have almost disappeared after the product was withdrawn in the 1970s.10 UTUC is very common in patients and families afflicted with Balkan endemic nephropathy (BEN).12 The incidence is highest in Croatia, Bosnia, Bulgaria, Romania and Serbia. Aristocholic acid (AA) contained in Aristolochia fangchi and Aristolochia clematis, plants endemic to the Balkans, is thought to play a role in the aetiology of UTUC. AA is the cause of Chinese herb nephropathy (aristocholic nephropathy or AAN), which is a rapidly progressing interstitial fibrosing renal disease with frequent urothelial malignancies.13 A high incidence of UTUC has also been described in Taiwan, especially in the population of the southwest coast, representing 20–25% of urothelial carcinomas in the region.
Most urothelial carcinomas of the renal pelvis are of high histological grade and present in advanced stages, whereas most bladder cancers present as low grade and stage.14,15
In the region of 40% of renal pelvis urothelial carcinomas can show unusual atypical features and metaplastic phenomena.15,16
Identification of Prognostic Factors
Prognostic factors are patient characteristics which predict the disease outcome including the chance of recovery or recurrence. Prognostic factors are significant and important in clinical practice as they can guide selection of treatment and the timing of surveillance. Identification of prognostic factors determining which patients can benefit from endoscopic versus radical nephroureterectomy, neoadjuvant or adjuvant chemotherapy and/or lymphadenectomy is urgently needed.
Management
Multiple treatment options are available for the management of patients with UTUC.
Open radical nephroureterectomy (ONU) with excision of an ipsilateral bladder cuff is the reference standard surgical treatment for UTUC in patients with a normal contralateral kidney regardless of the location of tumour in the upper tract. Nephroureterectomy can be performed by an open, laparoscopic (LNU) or hand-assisted technique (HARU). Resection of the distal ureter and its orifices is performed because it is a part of the urinary tract with especially high risk of recurrence and has been reported to be beneficial
Laparoscopic nephroureterectomy (LNU) is increasingly used in the management of UTUC.
Several studies have compared perioperative outcomes between ONU and LNU. LNU generally results in less blood loss, shorter hospital stay, decreased analgesic intake, a shorter interval to oral intake and decreased interval to convalescence, with no significant difference in the rate of perioperative complications.
Regarding oncological outcomes of ONU compared with LNU, emerging data support at least equivalent outcomes for LNU and ONU. In a prospective randomised trial, Simone et al. showed improved disease-specific and metastasis-free survival for ONU in patients with stage T3 tumours and high-grade disease. Long-term outcome data are awaited. ONU might improve cancer control in patients with high-grade/stage ureteral tumours. Port-site metastases following laparoscopic surgery for UTUC have been reported, which is another factor to be considered.
Traditionally, endoscopic management of UTUC was reserved for patients with solitary kidneys, bilateral synchronous tumours, baseline renal insufficiency, or comorbid diseases that preclude abdominal surgery. Recently, the use of endoscopic treatment via the retrograde ureteroscopic approach for patients with UTUC, normal renal function and low-grade, low-volume disease has been advocated, so long as patients are able to accept strict surveillance protocols. Opponents of ureteroscopic management argue that any delay in surgery might adversely affect prognosis but there is evidence suggesting that ureteroscopic biopsy/ablation before surgery does not adversely affect the outcomes compared with immediate surgery. Studies with longer follow-up are needed to assess the oncological efficacy of ureteroscopic treatments of UTUC.
Topical immunotherapy in addition to endoscopic management can be administered directly through a ureteral catheter or antegradely via a nephrostomy tube. Topical immunotherapy with BCG and chemotherapy with mitomycin C have a clear role in patients with bladder urothelial carcinoma; the data are not so clear for UTUC.
Consensus has not been reached regarding the therapeutic benefit of regional lymphadenectomy (LND) for UTUC. There is much clinical data supporting lymphadenectomy for bladder cancer but to date evidence with respect to lymphadenectomy for UTUC is mixed. Kondo et al. reported that preoperative imaging accurately identified lymph node positive patients in <50% of cases.17 This means that some patients might be understaged and undergo suboptimal treatment without lymphadenectomy. Lughezzani et al. found no difference in cancer-specific mortality in patients with UTUC undergoing surgery with and without lymphadenectomy.18 However, other studies have shown improvement in cancer-specific survival and disease-free survival in patients with stage T2 or greater disease who underwent lymph node dissection. Lymphadenectomy during radical NU remains controversial. Additional studies and the use of standardised dissection templates will help define the role of lymphadenectomy in the treatment of UTUC.19
Chemotherapeutic regimens for patients with advanced UTUC are based on those used for bladder urothelial carcinoma, of which cisplatin-based regimens are the most commonly used. In a large multicentre retrospective study on 140 patients, with locally advanced, node-positive disease and metastases, adjuvant chemotherapy with cisplatin-based regimens did not have any effect on overall or cancer-specific survival compared with the patients who did not undergo chemotherapy.
Biopsy
For patients with suspected UTUC found by diagnostic imaging, ureteroscopic biopsy provides histopathological confirmation and information as to the histological grade of the UTUC, which is one of the best predictors of pathological stage, tumour recurrence and overall survival. The diagnostic accuracy of ureteroscopic biopsy for UTUC is high but recent work has shown that ureteroscopic biopsy of upper tract lesions tends to underestimate the tumour grade when compared with the grade determined by histopathological analysis of the nephroureterectomy specimen. If ureteroscopic biopsy underestimates tumour grade, this may go some way in explaining the failure of some endoscopic management of UTUC. A high grade at biopsy could be used as supporting radical surgery.
Imaging Upper Tract Urothelial Carcinoma
Diagnostic imaging plays a significant role in diagnosing and staging of UTUC. Previously, multiple imaging investigations were common with ultrasound, excretory urography and retrograde pyelography. Currently, CT urography is the most common imaging technique for imaging UTUC and MR urography is used only when CT urography is contraindicated. Clinical presentation of UTUC is usually with haematuria and so the imaging techniques used must not only be highly accurate for diagnosing UTUC but also be able detect other diseases responsible for haematuria.
Definition of CT Urography
CT urography is a developing diagnostic imaging technique made possible by recent advances in CT technology. It is defined as a CT examination of the kidneys, ureters and bladder with at least one series of images acquired during the excretory phase, after administration of intravenous contrast medium.20
Indications and Contraindications for CT Urography
Early reports of CT urography for detecting urinary tract abnormalities including stones,21–24 renal masses25–28 and UTUC have been very encouraging.29–35 The first set of guidelines from the Upper Urinary Tract Imaging Group of the European Society of Urogenital Radiology was published in 2008.20
The indications for CT urography are controversial and consensus has still not been reached.33 The principal indication is the investigation of haematuria suspected to be due to UTUC. Other indications will not be discussed further here. A working knowledge of the indications for a particular diagnostic test is essential before requesting the said test, but there are three other key pieces of information that should be understood in order to avoid requesting redundant diagnostic tests. First, the clinician should use existing clinical knowledge of the patient to estimate the probability that the patient has the disease in question (the pretest probability). Secondly, the clinician should be aware of the sensitivity and specificity of the diagnostic test, and thirdly, the physician must consider whether the results of the test will affect the patient’s management.36 Contraindications are few but centre around whether iodine-based contrast or radiation should be avoided.37
Optimisation of CT Urography Technique
There are three main protocols for the intravenous administration of contrast medium which depend on the number of boluses of contrast used, single, double or triple bolus.38
The single bolus protocol is optimised for patients with haematuria at high risk of UTUC, renal masses and stones. This protocol consists of three series of image acquisition: an unenhanced series optimised for detecting stones, a nephrographic series optimised for detecting renal masses and an excretory series optimised for detecting UTUC.
The double bolus protocol involves a slightly lower radiation dose than the single bolus protocol. It uses less contrast medium than the single bolus protocol for demonstration of the nephrographic and excretory phases and so may not produce such an intense nephrogram for demonstration of renal cell carcinoma or excretory series for diagnosing UTUC. If the unenhanced series is excluded, and the enhanced nephrographic and excretory phases are acquired in one series, a low-dose protocol is achieved which is commonly used for the follow-up of patients with known bladder cancer or UTUC.32,39,40
The triple bolus protocol is used for the assessment of living related kidney donors and patients undergoing percutaneous nephrolithotomy.39 Although use of a triple bolus protocol for investigating haematuria has been described,40 it is currently not recommended for routine use because the volume of contrast agent available for the excretory phase is reduced by bolus splitting, which is likely to compromise diagnostic accuracy.
Many studies have evaluated techniques aimed at promoting complete opacification of the urinary tract during CT urography, in order to optimise the contrast between urine and urothelial disease. Currently there is no consensus regarding the optimum technique. A number of manoeuvres have been suggested that are often interrelated in a complex fashion so that adjustment of one will impact on the others. Such manoeuvres include oral administration of water, intravenous furosemide or saline to encourage urine flow, abdominal compression, varying the timing of the image acquisition, acquiring a second series of images in the excretory phase, increasing the volume of contrast injected and encouraging patient movement (walking or log rolling on the CT table) to promote mixing of contrast agent and urine.
Quality Control
Continuous audit of opacification is required to maintain the standard of CT urography from day to day. All users of CT urography should employ comparable scoring systems. In one such scoring system, the urinary tract is divided into four anatomical components (collecting system and renal pelvis; abdominal ureter; pelvic ureter; and bladder), and each is given a score of 0 (no opacification), 1 (partial opacification) or 2 (complete opacification). The bladder is scored slightly differently, with 0 representing no opacification, 1 is used for regions of interest within the bladder at less than 100 HU, and 2 corresponds to all regions of interest in the bladder over 100 HU. Only by using a similar scoring system across different centres will it be possible to accurately compare different CT urography techniques.38
Radiation Dose Optimisation Strategies
There has been much recent interest in radiation dose optimisation strategies.41,42 There are many ways to reduce radiation exposure from CT while maintaining diagnostic accuracy. Such methods may be considered in three groups: reducing radiation exposure before the examination, during the examination and after the examination. The most effective way to reduce radiation exposure is to not perform the examination. Scrutiny of examination appropriateness is essential. Once the decision has been made to perform CT, there are many available strategies to reduce radiation exposure; these include the use of size-dependent protocols, automated tube current modulation, reduction of the number of passes, reduction in duplicate coverage, reduction of mAs and kVp where possible, optimisation of intravenous contrast medium administration and possible use of external shielding. Radiation exposure can also be reduced with better post-processing methods to decrease noise, smoother kernels for reconstruction, reconstruction at larger slice thickness and iterative reconstruction.43
Diagnostic Accuracy of CT Urography for UTUC
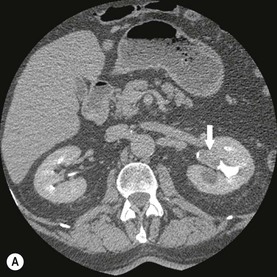
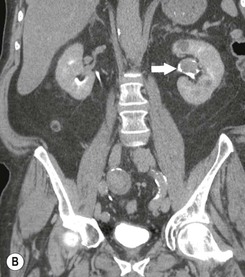
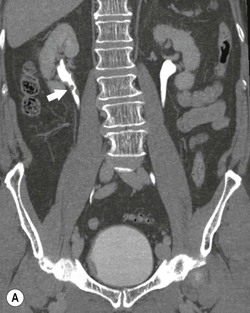
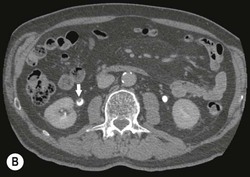
Diagnosing UTUC ultimately depends on detection of small filling defects in the collecting system of the kidney and ureter (Figs. 38-1 and 38-2). Such tumours may be pedunculated or sessile, invasive or non-invasive, local or metastatic.32
The reasoning for using CT urography for the investigation of haematuria ultimately depends on the high diagnostic accuracy of the excretory phase series in identifying urothelial cancer. Other component image series, unenhanced and nephrographic, have high diagnostic accuracy for stones and renal masses, but by definition they do not provide CT urography. To justify its use in patients with haematuria, CT urography must be as good if not better, in terms of diagnostic accuracy and patient acceptability, than the alternative techniques for imaging UTUC. A number of studies have evaluated the accuracy of CT urography for UTUC.30,44–49
Although it is conceptually simple to design a study that compares two imaging techniques in the same patients, in practice it is very difficult to justify the increased radiation dose that such patients would receive. Studies using surrogate comparisons are now commonly reported, which require careful scrutiny to ensure that optimum CT urography is compared with optimal IVU to mimic genuine clinical practice. It is also important to ensure that there are a sufficient number of patients with UTUC in each clinical cohort for an assessment of diagnostic accuracy for UTUC to be meaningful.50
In 2010, Wang et al.51 performed a retrospective study of adult patients with haematuria who underwent both CT urography and IVU over a 2.5-year period in a single institution; 19 of 60 patients had UTUC. The sensitivity, specificity and accuracy of IVU were 0.750, 0.860 and 0.849, respectively, compared with 0.958, 1.000 and 0.996 using CT urography for UTUC. The authors concluded that CT urography should be the first-choice non-invasive imaging technique for diagnosing UTUC in patients with haematuria. A similar study was reported by Jinzaki et al. in 104 patients with haematuria, 46 of whom had UTUC.49 Per-patient sensitivity, specificity and overall accuracy for the detection of UTUC with CT urography (93.5, 94.8 and 94.2%, respectively) were significantly greater than the corresponding values for excretory urography (80.4, 81.0 and 80.8%; P = 0.041, 0.027 and 0.001, respectively.49 Although the designs of these studies are theoretically imperfect, the results suggest that the diagnostic accuracy of CT urography is greater than IVU for upper urinary tract disease, especially UTUC.
Several retrospective studies report on the diagnostic accuracy of CT urography for UTUC,30,45–48 against the reference standard of histopathology, cytology and clinical follow-up. Despite the variation in CT urography technique, most report high sensitivities and specificities for UTUC. Many studies using a double bolus protocol give the majority of the contrast volume in the second bolus, a move that is likely to reduce the opacification scoring of the upper tract in the excretory phase as much of the contrast agent will not have been excreted into the collecting systems and ureter at the time of image acquisition.
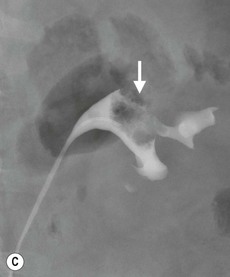
In 2007, Cowan et al. compared the diagnostic accuracy of CT urography and retrograde ureteropyelography for diagnosing UTUC.44 The clinical cohort consisted of a selected group of 106 patients who presented with haematuria and initially underwent IVU and flexible cystoscopy. Patients with equivocal or positive IVU results and those with negative IVU and flexible cystoscopy results but with persistent haematuria were investigated with CT urography and retrograde ureteropyelography. The reference standard was histopathology from biopsy or resected specimens and 3–5-year clinical follow-up. The sensitivity, specificity, positive predictive value and negative predictive value of CT urography for diagnosing UTUC were 0.97, 0.93, 0.79 and 0.99, respectively. For retrograde ureteropyelography, the sensitivity, specificity, positive predictive value and negative predictive value were 0.96, 0.97, 0.87 and 0.97, respectively. Thus the diagnostic accuracy was similar for CT urography and retrograde ureteropyelography, which is an important result given that retrograde ureteropyelography is often regarded as the gold standard for imaging UTUC. The authors concluded that the quantitative evidence provided by the study validated the use of CT urography for diagnosing UTUC.44
Diagnostic Imaging Strategies for UTUC
Conventional diagnostic imaging pathways for haematuria—including ultrasound, IVU and CT urography—are complicated and lengthy. The principal aim of any new diagnostic strategy must be to simplify and shorten the time from presentation to diagnosis. The diagnostic accuracy of CT urography for urinary tract stones and solid renal masses surpasses that for ultrasound and IVU as described in the previous section. This means that CT urography has the potential to be a single replacement test for ultrasound and IVU. New evidence relating to the diagnostic accuracy of CT urography for diagnosis of UTUC suggests CT urography might also be the technique of choice for imaging the urothelium for suspected urothelial carcinoma.44,47,49,51–53
Whether all patients with haematuria should undergo CT urography, or whether there are subgoups of patients who are more likely to benefit from CT urography than others, remains under investigation. Some notable features of CT urography mean that it should be targeted towards a select patient population. These include the length of time required in the CT suite, the increased radiation dose compared to other CT studies, and the large bolus of intravenous contrast medium administered.
The ideal initial diagnostic imaging test should have high sensitivity and specificity for diseases at high prevalence in the test population. Deciding what constitutes a high prevalence is not just a clinical decision but also depends on economic and political factors. There is a 4.4–22 times greater prevalence for UTUC and a 1.8–5.1 greater prevalence for stones in a study using CT urography rather than US and/or IVU as the initial imaging investigation.38,54–56 Although the populations cannot be exactly age matched in the three studies, the difference in prevalence is striking and is most likely to reflect that greater diagnostic accuracy of CT urography compared with ultrasound and IVU for investigating haematuria.
The prevalence of specific underlying diseases responsible for haematuria can be predicted with various risk factors. The two most readily identifiable risk factors that reflect the prevalence of urothelial carcinoma are the presence of visible or non-visible haematuria and increasing patient age. This knowledge can be used clinically for stratification into groups of patients at low risk and high risk for urothelial carcinoma.
The high-risk group consists of patients 50 years with visible haematuria and urinary tract infection excluded. UTUC is the most prevalent disease in this population, which justifies initial investigation with CT urography.
The low-risk group comprises patients >40 years with non-visible haematuria and those 50 years old with either non-visible or visible haematuria. The disease with the greatest prevalence in patients with non-visible haematuria under the age of 50 years is medical renal disease,57 so renal tract ultrasound is recommended as the initial imaging test in these patients. Ultrasound can elucidate the presence, position and outline of the kidneys, cortical thickness, and the presence of large stones, large renal masses and hydronephrosis. Calculi represent the most common cause of haematuria in patients under the age of 50 years with visible haematuria and those older than 50 years of age with non-visible haematuria with urinary tract infection excluded. The prevalence of UTUC is very low in these patients. Given that unenhanced CT has the highest sensitivity for upper urinary tract stones, it is recommended as the initial imaging investigation in these patients. Unenhanced CT can detect small stones, hydronephrosis, hydroureter and some renal masses, upper tract urothelial cancers and bladder cancers.
For patients of any age in the low-risk group with persistent non-visible haematuria and normal imaging investigations, repeat imaging is only justified if there is a significant change in the risk score: for example, if urinary tract symptoms or visible haematuria are reported. The optimum diagnostic imaging strategy for patients at high risk for urothelial carcinoma consists of initial CT urography as a replacement for conventional upper tract imaging techniques52 and as a triage test for bladder assessment. For patients at low risk of UTUC, ultrasound and unenhanced CT of the kidneys, ureters and bladder should be used instead.20
Problems and Solutions
The principal problems associated with CT urography for investigating haematuria are reader error, false-positive diagnoses and increased radiation dose. Other problems that are also important but are considered secondary issues include the length of time the patient is required to be in the CT room for the complete examination, availability of CT, cost compared with other imaging techniques, the need for special image-viewing hardware and software for optimum reporting, the diagnosis of unexpected extragenitourinary disease, the requirement for a quality assurance programme and the overutilisation of CT urography for inappropriate indications.
There are many ways to reduce reader error, but perhaps the best method of teaching radiologists to read CT urography is to introduce a formative teaching programme designed to simulate clinical reporting. Reader training, interpretation and certification has already been employed for CT colonography.56,58 Various diagnostic accuracy studies have revealed that the positive predictive value of CT urography for UTUC is low, ranging from 0.50 to 0.82, which currently precludes the notion of referring patients for curative surgery as soon as they are diagnosed. A list of false-positive diagnoses is given in Table 38-1. Therefore, UTUC diagnosed with CT urography should be biopsied (by ureteroscopy or retrograde ureteropyelography), guided for histopathological confirmation of the diagnosis before proceeding to surgery.59
TABLE 38-1
False-Positive Diagnoses for UTUC
| 1 | Clot |
| 2 | Debris |
| 3 | Kink of the ureter (accentuated by respiration) |
| 4 | Fibroepithelial polyp |
| 5 | Injury to the ureter following passage of a stone, stent placement or ureteroscopy |
| 6 | Ureteritis cystica |
| 7 | Flow artefact following furosemide administration or layering effects |
| 8 | Unusual-looking papilla, a normal variant |
| 9 | Nephrogenic adenoma |
| 10 | Amyloid |
| 11 | Renal cell carcinoma |
| 12 | Lymphoma |
| 13 | Vascular impression |
| 14 | Inflammation, fibrosis |
Overutilisation of high-technology imaging services such as multidetector CT, MR imaging and PET-CT is defined as performing imaging procedures that are unlikely to improve patient outcome.60 Healthcare systems in which there is a fee-for-service payment process, self-referral practices and defensive medicine may be vulnerable to the overutilisation of imaging, which can be responsible for unnecessary healthcare costs and frequently exposes patients to unnecessary radiation. CT urography requires a higher dose of radiation than excretory urography, which can only be justified by its increased diagnostic accuracy in the correct clinical setting.61 Individual patient radiation doses can be minimised by close attention to optimising image acquisition parameters and by using CT dose-reducing techniques.62 The use of referral guidelines that incorporate appropriateness criteria based on objective clinical evidence and comparative effectiveness research makes an important contribution to reducing overutilisation. Further comparative effectiveness research of imaging technology is called for as a deterrent to overutilisation of medical imaging.
Conclusions and Summary
The principal indication for CT urography is the investigation of haematuria. Rapid developments in multidetector CT technology make multiplanar review of isotropic data sets possible providing high-resolution CT urography studies. The high diagnostic accuracy of unenhanced CT for calculi, the nephrographic phase CT for renal masses and the excretory phase CT for urothelial cancer, compared with other techniques, makes CT urography the preferred initial imaging investigation for patients presenting with haematuria. To ensure maximum diagnostic accuracy, attention to detail of the CT urography technique is important. For patients at high risk for BCa and UTUC, CT urography should be used as a triage test before cystoscopy and as the initial imaging test for examination of the upper urinary tract. By careful attention to indications and technique, the radiation dose of CT urography can be optimised for those patients with haematuria who are at high risk of BCa and UTUC. Formative teaching programmes simulating clinical reporting are suggested as the preferred method of teaching radiologists to read CT urograms and to reduce reader error. As the positive predictive value of CT urography for UTUC is low, UTUC diagnosed with CT urography should be biopsied (by ureteroscopy or retrograde ureteropyelography) guided for histopathological confirmation of the diagnosis before proceeding to surgery.
In the future, MR imaging and dual-energy CT may have an important role in imaging UTUC. MR urography has some advantages over CT urography in that it does not use ionising radiation, but currently the spatial resolution of MR urography falls below CT urography, which is important when looking for small urothelial lesion in the upper urinary tract.63–65 Dual-energy CT is currently being evaluated in clinical practice to determine its role in urinary tact imaging, and to address the issue of additional radiation exposure associated with dual-energy imaging.66
In conclusion, CT urography is recommended as the initial imaging test for patients presenting with haematuria who are at high risk of urothelial cancer, and specifically for imaging adult patients over 50 years of age presenting with visible haematuria, once infection is excluded.
Bladder Cancer
Introduction
Bladder cancer is the fourth most common cancer in the developed world. The incidence of bladder cancer increases with increasing age. While genetic factors are also likely involved, bladder cancer is believed to develop in many individuals as a result of excretion of ingested carcinogens into the urine. Accordingly, there are a number of environmental risk factors for bladder cancer, the most substantial of which is smoking.67 It has been estimated that cigarette smoking may be responsible for 50–60% of bladder cancers. Another 20% of bladder cancers are likely the result of occupational exposure (which result from working in the following areas: textiles or petrochemicals, bus/truck driving (due to diesel fumes), firefighting, hairdressing, plumbing and printing). There is also an increased risk of bladder cancer in patients who have been exposed to non-steroidal anti-inflammatory medication and to the chemotherapeutic agent cyclophosphamide. Primary bladder neoplasms are typically epithelial in origin, with urothelial cancer being the most common.
Classification of Urothelial Cancers
By Growth Pattern
Urothelial cancers are generally divided into two major groups: those that are superficial (which do not invade the muscular layer of the bladder wall or the muscularis mucosa) and those that are invasive (which extend into the muscularis mucosa). Seventy to 80% of bladder tumours are superficial neoplasms, usually limited to the mucosa.68 Superficial neoplasms are papillary neoplasms about 70% of the time. Papillary urothelial neoplasms are usually of low grade and have a good prognosis.69 The remaining 30% of superficial urothelial tumours are flat neoplasms and are classified as carcinoma in situ. Flat tumours are typically of higher grade and are more likely to become invasive, if left untreated, with approximately 80% eventually progressing to invade the muscular layer of the bladder wall.67 Some superficial tumours extend into the lamina propria. In general, superficial bladder tumours are treated topically by transuretheral resection (TURBT) and/or instillation of immunological (with bacille Calmette-Guérin (BCG)) or chemotherapeutic agents. Unfortunately, there is a 70% likelihood that any patient with a superficial bladder cancer will have a recurrence in the bladder within 3 years of presentation, with 10–20% of these recurrences being more aggressive.68 About 20–30% of bladder neoplasms are muscle invasive at the time of presentation. Surgical resection (cystectomy) is required for cure of these patients.
By Histology
While most bladder cancers are composed entirely of transitional cells, a substantial minority (up to 40%) of urothelial cancers of the bladder, which were previously classified as transitional cell carcinomas, contain focal areas of atypical or divergent histology, with atypical features in addition to transitional cells, consisting of areas of poor differentiation, squamous cells, sarcomatoid cells, adenomatous cells and small cells.70–72 Additional atypical histologies include clear cell, glandular cell, lymphoepithelial, micropapillary, plastmacytoid and neuroendocrine components.70,73
The presence of areas of atypical histology has been shown to affect patient prognosis poorly, as transitional cell tumours with divergent histology tend to behave more aggressively, both locally71 and in terms of the likelihood of developing distant metastatic disease.72 In a study by Shinagare et al., urothelial tumours containing areas of atypical histology were significantly more likely to spread to the peritoneum, with only 6 of 94 metastatic pure transitional cell cancers involving the peritoneum, in comparison to 18 of 56 neoplasms with divergent histology.72
Pure squamous cell carcinomas account for only 6–8% of all urothelial cancers.74 These irritative cancers are more commonly encountered in areas where schistosomiasis is endemic or in patients with chronic urinary tract infections or bladder calculi. Bladder adenocarcinomas are even less common, being responsible for no more than 2% of all bladder cancers.69,74 Squamous cell carcinomas and adenocarcinomas of the bladder will be discussed in more detail in later sections of this chapter.
By Grade
Grading of urothelial neoplasms is important, as it can affect treatment and predict patient outcome. According to the recent World Health Organisation classification,75 papillary urothelial neoplasms are differentiated into four grades:
2. Papillary urothelial neoplasms of low-malignant potential (PUNLMP) are of slightly higher grade. PUNLMP cells may have uniformly enlarged or elongated nuclei and rare mitotic figures. At the present time, some do not classify these neoplasms as benign or malignant.76 PUNLMPs have an extremely low likelihood of recurrence and little to no risk of progression.76
Clinical Detection
The most common presentation of bladder cancer is painless haematuria, which may be macroscopic or microscopic.77 This symptom is not specific, as it is frequently encountered in patients with cystitis and benign prostatic hyperplasia. In fact, most patients with haematuria do not have bladder cancer.78 It is estimated that 12–20% of patients with macroscopic haematuria have bladder cancer,78 while a much lower percentage of patients with microscopic haematuria have bladder cancer. Other less common symptoms in patients with bladder cancer include dysuria, frequency and/or urgency.
While urine cytology may be positive in up to 90% of patients with carcinoma in situ, cytology is much less sensitive in patients with superficial papillary bladder cancers.67 Thus, negative urine cytology cannot be utilised to rule out a diagnosis of bladder cancer definitively.
The gold standard for bladder cancer identification is cystoscopy, although, on occasion, some bladder neoplasms can be missed during this procedure. Initial cystoscopies can be performed as an outpatient office procedure using a flexible cystoscope. In the past, cystoscopy was performed using white light; however, some investigators have recently shown that fluorescent-light cystoscopy (performed after staining the bladder wall with fluorescent dyes) has higher sensitivity in detecting bladder cancer.79
Imaging Detection
The ability of imaging studies to detect bladder cancer traditionally has been considered to be less important than their ability to detect upper tract urothelial cancers. This is because, as has already been mentioned, flexible cystoscopy is very sensitive and is an easily performed outpatient procedure. In comparison, upper tract evaluation by urologists requires ureteroscopy, a more involved procedure (usually performed under general anaesthesia). For this reason, the upper urinary tracts are frequently assessed with imaging studies.
Conventional excretory urography and cystography have long been known to have limited efficacy in detecting bladder cancers. In one study, only 60% of bladder cancers could be identified on excretory urograms.78 With the recent emergence of CT urography and MR urography, however, imaging detection of bladder tumours has improved dramatically, with identification of even tiny urothelial bladder lesions now being possible.53,81
Technique of CT Urography and MR Urography
Both CT urography and MR urography are usually performed utilising thin-section reconstructed images obtained before and after the intravenous administration of iodinated (for CT) or gadolinium-based (for MRI) contrast media. At least one series of images is routinely obtained during the excretory phase of contrast material enhancement (which occurs 5–15 min after contrast material injection begins), at which time contrast material has usually been excreted into the renal collecting systems and ureters and has opacified the bladder. It has been generally accepted that sensitivity in detecting urothelial neoplasms on CT urography is maximised when excretory phase images are obtained.81,82 This belief has recently been challenged in several studies,83,84 where it has been observed that detection of upper and lower tract urothelial neoplasms is actually as good or greater when images are acquired 60 s after the initiation of the contrast media injection (during the corticomedullary phase of renal enhancement), at which time abnormally increased enhancement of even small urothelial neoplasms against a background of unopacified urine can be detected. The increased enhancement of urothelial cancers facilitates differentiation of these lesions from normally enhancing urothelium, urothelium that is thickened as a result of benign disease, or non-enhancing non-calcified filling defects within the urinary tract, such as blood clots, mucus, or fungus balls. In fact, it is most likely that the CT or MR urographic approach most sensitive for detecting bladder cancer is one where both early enhanced (corticomedullary phase) and delayed enhanced (excretory phase) series are obtained.
The above recommendations notwithstanding, a variety of contrast material injection techniques have been utilised for CT urography. Some investigators prefer a single bolus, with at least two series of contrast-enhanced images obtained (at least one during the corticomedullary and/or nephrographic phases, with the latter usually beginning 100 s after the initiation of the contrast material injection, and another during the excretory phase of contrast material enhancement). Some also obtain an additional enhanced series during the arterial phase. Another widely used technique is split-bolus injection, with each of two boluses administered separately, usually at least 5–10 min apart, before obtaining any contrast material-enhanced images.85 Image acquisition of a single contrast-enhanced series is then performed at a time when the first bolus has been excreted into the renal collecting systems, ureters and bladder, while the second bolus is still opacifying the renal parenchyma and the bladder wall. Gadolinium-based contrast material injection during MR urography is generally performed as a single bolus, with multiple series of post-injection images obtained (often including the corticomedullary, nephrographic and excretory phase phases).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree