Uterine leiomyomas or fibroids are the most common uterine and gynecologic neoplasm, with a reported incidence ranging from 20% to 40%. Fibroids are benign tumors that arise from the smooth muscle of the uterus and may be submucosal, intramural, or subserosal in location. Although their etiology is unclear, a multifactorial process linked to hormonal dysregulation and genetic factors is favored. Clinically, uterine leiomyomas may result in a variety of symptoms, including menorrhagia, metrorrhagia, bulk symptoms, and pelvic pain prompting women to seek therapy. Their position, size, and direction of growth affect the type and degree of symptoms. Submucosal and exophytic submucosal lesions increase the endometrial surface, resulting in a larger potential area for bleeding, whereas subserosal and intramural lesions alter uterine contractility and may exert mass effect on surrounding structures.
Uterine artery embolization (UAE) for the treatment of symptomatic fibroids was introduced by Ravina et al. in 1995 and has since proven to be a safe and effective treatment option. UAE was performed before 1995 for other indications, including posttraumatic, postpartum, or postsurgical bleeding, embolization of pelvic arteriovenous malformations, and bleeding ectopic pregnancies. In patients with fibroids, UAE results in the infarction of fibroids by injecting a particulate embolization agent into the main uterine arteries. The hypervascular nature of fibroids causes the embolization agents to preferentially target the fibroids, with relative sparing of the normal myometrium, ultimately resulting in infarction, coagulation necrosis, retraction, and shrinkage of the fibroids. The vessels supplying the fibroids are preferentially occluded because they are larger and have increased flow than normal myometrial branches.
Before the advent of UAE for the treatment of symptomatic uterine fibroids, surgical hysterectomy or myomectomy was the mainstay in the treatment of fibroids. Compared with surgical treatment of fibroids, UAE is associated with decreased morbidity, shorter hospitalization, and uterine preservation.
Medical and Surgical Treatment Options for Symptomatic Patients
Medical therapy including the use of nonsteroidal antiinflammatory drugs or acetaminophen may be an effective first-line treatment option in reducing the pain associated with fibroids, although these agents do not decrease uterine bleeding. Several other medical options exist, including androgenic steroids, the progesterone-blocking agent mifepristone (RU-486), and gonadotropin-releasing hormone (GnRH) agonists including leuprolide (Lupron), and GnRH antagonists such as ganirelix, all of which have been shown to reduce uterine and fibroid volume and bleeding. The use of progesterone-releasing intrauterine devices has also been shown to decrease bleeding. However, these medical options are accompanied by significant side effects or potential risks. The benefits of GnRH agonists, for example, are limited by a multitude of side effects that occur in the wide majority of patients and the compounding risks associated with their long-term use.
More invasive options for the treatment of women with symptomatic uterine fibroids include hysterectomy, myomectomy, endometrial ablation (when menorrhagia is the primary complaint and there are no other endometrial lesions or anatomic abnormalities), and UAE. More aggressive intervention in women with uterine fibroids is generally indicated only when symptoms are severe enough and become unacceptable to the patient. Serious medical conditions, such as severe anemia or ureteral obstruction, often need to be treated surgically.
Hysterectomy involves major surgery and has associated risks. The average hospital stay is 5 days with a recuperation time of 3 months. With UAE the recuperation time is typically much shorter, and the average hospital stay is only 1 night. In a study of 459 women who underwent hysterectomy and 649 women who underwent UAE published by Dutton et al., fewer complications were experienced by the women in the UAE cohort, in particular the more severe and major complications (19% for UAE vs. 26% for hysterectomy). Hysterectomy is considered a definitive treatment, however, because there should be no recurrence of fibroid-related symptoms. Although more women in the hysterectomy cohort reported relief from fibroid symptoms (95% vs. 85%) and feeling better (96% vs. 84%), only 85% would recommend hysterectomy to a friend compared with 91% in the UAE group.
Patient Selection for Uterine Artery Embolization, Indications and Contraindications
UAE for the treatment of uterine fibroids is indicated in the setting of incapacitating, abnormal menstrual bleeding, pain and/or bulk symptoms when uterine preservation and/or avoidance of surgical procedures is desired, and/or medical or surgical therapy has failed.
Absolute contraindications to UAE include a viable pregnancy, active infection, and suspected adnexal or uterine malignancy. A history of an allergic reaction to iodinated contrast material, renal insufficiency, and coagulopathy are considered relative contraindications to UAE. Additional, relative contraindications have been reported and include a uterine size greater than 24 weeks and the presence of a uterine fibroid greater than 10 cm. Although large, pedunculated fibroids at risk for detachment have been reported as a relative contraindication, a study of 196 patients suggested that lesions with a stalk diameter of greater than 2 cm can be successfully treated with UAE. Smeets et al. found that embolization of pedunculated fibroids is safe and effective and that the risk for serious complications is probably rare. It has been suggested that patients with intracavitary fibroids, broad-ligament fibroids, and cervical fibroids may be less than ideal candidates for UAE. Because the preservation of fertility cannot be guaranteed with UAE, the desire for future pregnancies should be carefully weighed and is potentially a relative contraindication. However, because uncomplicated pregnancies have been reported to occur after UAE, it may represent a potential treatment option for patients who are not myomectomy candidates and wish to preserve their fertility.
Preprocedural Imaging
Because the effectiveness of UAE in the treatment of uterine fibroids is influenced by a number of anatomic factors, preprocedure imaging is essential. At the time UAE emerged as an alternative for the treatment of women with fibroids, sonography was the primary imaging modality for the preprocedural workup and follow-up evaluation of these patients. Pelvic magnetic resonance imaging (MRI) now plays an increasing role in preprocedural imaging and is currently favored at many institutions. MRI has been shown to assess fibroid location, size, and number with a much greater accuracy than sonography. MRI can also assess for the presence or absence of fibroid perfusion and vascularity and also allows for better identification of diffuse or focal adenomyosis compared with sonography. One study showed MRI changed the treatment plan in 18% of patients with presumed symptomatic uterine fibroids, in many instances allowing for the diagnosis of other pelvic pathology including adnexal masses and adenomyosis. Disadvantages of MRI compared with sonography include higher cost, longer examination time, and less-detailed evaluation of the endometrial lining.
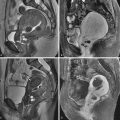
On imaging, uterine fibroids appear as well-circumscribed masses with well-defined margins. They can be categorized according to their location in the uterus as submucosal, subserosal, or intramural. The term intracavitary is used when a submucosal fibroid is located almost entirely within the endometrial canal. On MRI, uterine fibroids are characteristically hypointense on T1- and T2-weighted sequences. In the absence of internal degeneration, they demonstrate avid enhancement on post–gadolinium sequences ( Figure 39-1 ). As fibroids increase in size, they may undergo internal degeneration that can change their MRI appearance. Hyaline degeneration is the most common type of degeneration. In this setting a fibroid may demonstrate heterogeneous T2 signal and decreased enhancement. Other types of degeneration that have been described include hemorrhagic, cystic, and fatty degeneration. Fibroids with hemorrhagic degeneration demonstrate increased T1 signal secondary to the presence of methemoglobin and typically will show minimal to no enhancement ( Figure 39-2 ). In the setting of hemorrhagic degeneration, fibroids show variable T2 signal depending on the age of hemorrhagic breakdown products. Cystic degeneration appears as T2 bright foci within the tumor similar to the signal intensity of fluid; after the administration of gadolinium, the cystic spaces will not enhance. Fatty degeneration of a fibroid to a lipoleiomyoma is rare. The presence of fat can be readily identified by MRI with use of fat-suppressed sequences. Malignant degeneration of uterine leiomyomas is rare and can be difficult to diagnose on MRI in the absence of metastatic disease. Tanaka et al. have reported that leiomyosarcoma should be considered if there has been rapid growth, more than 50% of a fibroid demonstrates high signal intensity on T2-weighted imaging, T1-hyperintense foci (of any size) are present, or if avascular pocket-like areas are seen on postcontrast images. In a recent study, however, Cornfeld et al. found poor accuracy of objective imaging criteria in distinguishing leiomyomas with atypical imaging features from more clinically significant uterine mesenchymal neoplasms such as leiomyosarcoma.


In evaluating the potential UAE candidate, several factors should be considered and noted on the preprocedural MRI. First, several studies have shown the size of the largest fibroid to impact the success ofUAE. A successful procedure is defined by symptomatic relief, fibroid volume reduction, and patient satisfaction. In a study of 200 patients, Spies et al. reported that a smaller baseline size and a submucosal location are most likely to result in a good outcome. Pelage et al. reported that large fibroid diameter (>10 cm) can be a predisposing factor for serious complications and that UAE should not be performed for fibroids larger than 10 cm. A more recent study of 47 patients, however, concluded that patients with large tumor size undergoing fibroid embolization are not at increased risk for complications, and successful outcomes can be obtained in these patients.
The position of the fibroids in the uterus should be noted on preprocedural MRI. Submucosal or intramural fibroids with a large submucosal component have been shown to have an increased risk for vaginal expulsion of necrotic tissue. This is associated with pain, bleeding, infection, and prolonged vaginal discharge and has been found to be the most common complication of UAE requiring hospitalization. Although the presence of submucosal fibroids does not necessarily exclude UAE as a therapeutic option, it does require that patients be appropriately informed of the risks associated with such positioning of fibroids. A recent study by Radeleff et al. found a low complication rate and high treatment satisfaction despite a high rate of fibroid expulsion (50%) in patients with a dominant submucosal fibroid.
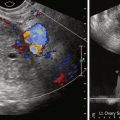
Pedunculated subserosal fibroids are generally considered a relative contraindication for UAE with significant variation among institutions ( Figure 39-3 ). Pedunculated fibroids are defined as tumors in which the diameter of the stalk is 50% narrower than the diameter of the fibroid. Rarely, pedunculated subserosal fibroids can separate from the uterus secondary to stalk necrosis and migrate into the peritoneal cavity after embolization. With migration into the peritoneal cavity, parasitization of the peritoneal blood supply, necrosis, or sepsis can occur. Intraperitoneal adhesions, chronic peritonitis, and protracted pain syndrome are additional reported complications. In one study by Katsumori et al., however, no serious complications after embolization for pedunculated subserosal fibroids with stalk diameters of 2 cm or greater were encountered, and successful outcomes were reported.

The presence of coexisting adenomyosis also poses a unique situation in the treatment of fibroids with UAE. In a study by Goodwin et al., adenomyosis was found in 50% of patients who required hysterectomy after UAE for inadequate response to therapy. A study evaluating patients with adenomyosis and fibroids, adenomyosis as the dominant disease, and adenomyosis alone undergoing UAE concluded that the presence of adenomyosis should not be a contraindication to embolization because most patients showed clinical improvement after undergoing the procedure. Nonetheless, coexisting adenomyosis should be noted before the procedure, and patients should be informed of a more variable response therapy.
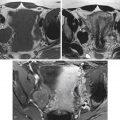
Pelvic MRI is highly accurate in diagnosing adenomyosis and differentiating it from uterine fibroids. Adenomyosis can be diffuse or focal. The diffuse form is more common. On MRI, one of the hallmarks of adenomyosis is thickening of the T2-hypointense junctional zone (>12 mm). If the junctional zone measures 8 mm or less, adenomyosis can be excluded with high specificity. T2-weighted bright foci classically seen within the thickened junctional zone represent ectopic endometrial glands, cystically dilated endometrial glands, or hemorrhagic foci ( Figure 39-4 , A ). T1-bright foci can also be seen and correspond to areas of hemorrhage. These foci may not enhance after gadolinium administration, but the majority of involved myometrium should enhance uniformly (see Figure 39-4 , B ). More focal adenomyosis or an adenomyoma may appear as an area of focal junctional zone thickening or a poorly defined mass with minimal mass effect on the endometrial canal (see Figure 39-4 , C ).

Magnetic Resonance Protocol
Preprocedure and postprocedure pelvic MRI examinations are obtained using the same protocol, which includes a combination of T1- and T2-weighted sequences in multiple planes. A 1.5-Tesla magnet using high-performance gradient systems and a phased array body coil is sufficient. MRI sequences obtained in our departmental protocol include axial and coronal half-acquisition rapid acquisition with relaxation enhancement (RARE), axial and sagittal T2-weighted RARE, and axial, sagittal, and coronal T1-weighted fat-suppressed two- or three-dimensional (3D) before and after contrast enhancement.
T1-weighted sequences provide an excellent overview of the pelvis. Nonenhanced T1-weighted fat-suppressed sequences are useful in demonstrating blood elements that can be seen in hemorrhagic foci in adenomyosis, hemorrhagic degeneration of fibroids, and even coexisting endometriosis. Gadolinium-enhanced T1-weighted fat-suppressed sequences are essential for demonstrating vascularity of the uterine fibroids. T2-weighted fast spin echo sequences should be obtained in at least two, and preferably all three, planes because they are useful in demonstrating zonal anatomy and assist in exact fibroid localization. A smaller field of view centered on the uterus should be used for T2 sequences ( Table 39-1 ).
| s equence | tr/te | f ield of View | Slice Thickness | Matrix |
|---|---|---|---|---|
| Scout | ||||
| Axial T1 | 649/12 | 30 cm | 6 mm, gap 1.8 | 160/51 |
| Axial HASTE T2 (non-BH) | 1000/64 | 30 cm | 5 mm, interleaved | 130/256 |
| Coronal HASTE T2 (non-BH) | 1000/64 | 30 cm | 5 mm, interleaved | 130/256 |
| Sagittal T2-TSE (SAT BAND, high resolution, non-BH) | 4200/125 | 22 cm | 4 mm, gap 1.0 | 225/256 |
| Axial T2-TSE (as above) | 4200/125 | 22 cm | 4 mm, gap 1.0 | 225/256 |
| PRE-GAD T1 FS GRE axial and sagittal (BH-17SEC) | 153/1.9 | 30 cm | 6 mm, gap 1.8 | 115/256 |
| POST-GAD axial T1 FS GRE (BH = 17 s, scanned immediately and at 60 s after contrast) | 153/1.9 | 30 cm | 6 mm, gap 1.8 | 115/256 |
| POST-GAD Sagittal T1 FS GRE (BH = 17 s, scanned at 90 s) | 153/1.9 | 30 cm | 6 mm, gap 1.8 | 115/256 |
| POST-GAD Coronal T1 FS GRE (BH = 17 s, scanned at 2 min) | 153/1.9 | 30 cm | 6 mm, gap 1.8 | 115/256 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree