Chapter 6 Valvular Heart Disease
Cardiac imaging in suspected valvular disease determines the involvement of the valves, the extent of the stenosis or regurgitation, and the hemodynamic consequence of the pressure or volume overload on the heart. It also evaluates associated conditions, such as aortic dissection or aneurysm and ventricular contractility and enlargement.
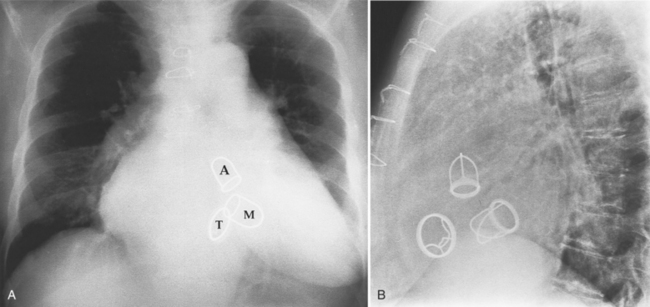
Fig. 6-1 illustrates the anatomic positions of the heart valves.
VALVULAR AORTIC STENOSIS
Chest Film Findings
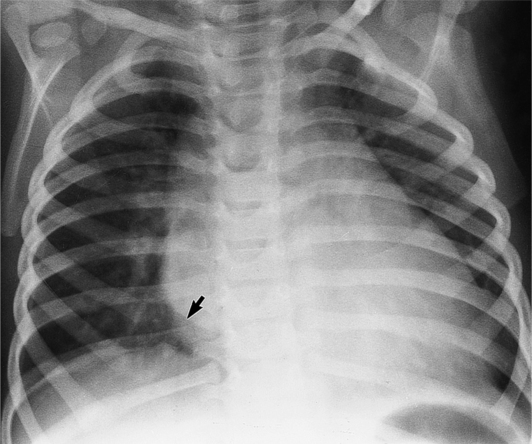
The chest film abnormalities depend on the age of the patient and the severity of the stenosis. In infancy, pulmonary edema with generalized cardiomegaly is the typical appearance of obstruction to blood flow at any point between the pulmonary veins, left heart, and aorta (Fig. 6-2). The adult heart is normal in size, and the lungs are clear unless there is left ventricular failure and dilatation (Fig. 6-3). Calcification in the aortic valve over age 40 years occurs in all types of aortic stenosis and clinically marks the stenosis as severe. Dilatation of the ascending aorta is frequent in aortic stenosis but correlates rather poorly with severity or with the site of the stenosis (one third of patients with subvalvular aortic stenosis have dilated aortas).
Imaging Features
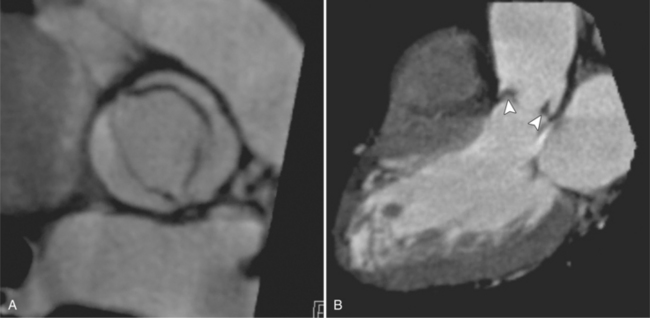
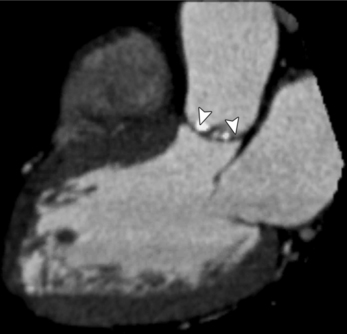
The preferred imaging modality for the dynamic assessment of aortic stenosis remains echocardiography. Cardiac-gated multidetector CT (MDCT) angiography is proving to be an excellent modality to detect and quantify aortic valve stenosis. Direct planimetry of the valve opening in systole correlates quite well to echocardiographic grades of aortic stenosis severity. The added advantage of MDCT is that it nicely demonstrates associated aortic root pathologies. Fig. 6-4 shows a case of mild aortic stenosis with the valve in short axis during systole (open) and diastole (closed). Fig. 6-5 shows the same valve in long axis during systole and diastole. Finally, MRI can be useful for aortic stenosis because it can accurately quantify peak aortic valve velocities and pressure gradients. The associated calcium on the valve makes MRI less useful to assess morphology because of the typical signal loss of calcified structures on gradient echo pulse sequences. A narrow orifice to the aortic valve is visible as a jet through the valve. The degree of aortic stenosis can be estimated by comparing the width of the jet with the diameter of the aortic annulus. When the jet is less than 15% of the diameter of the annulus, there is severe aortic stenosis. However, it may also be present when the width of the column of blood through the valve appears to be the same size as the annulus. This latter finding is typical of degenerative aortic stenosis in the elderly and in many cases of rheumatic involvement of the aortic valve. In the former, the valve orifice is irregularly eccentric without commissural fusion, allowing the stream of contrast material adjacent to the three commissures to project over the entire width of the aorta. Calcification may be so extensive that the leaflets are akinetic.
Box 6-1 lists the causes of aortic stenosis.
Congenital Valvular Aortic Stenosis
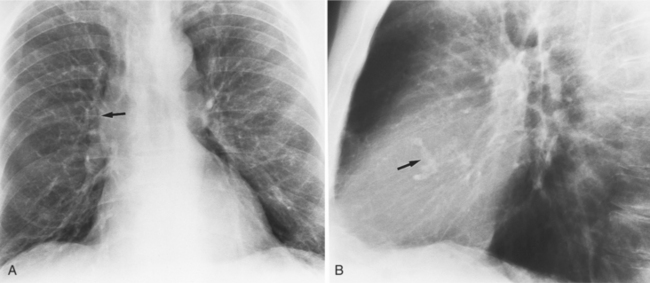
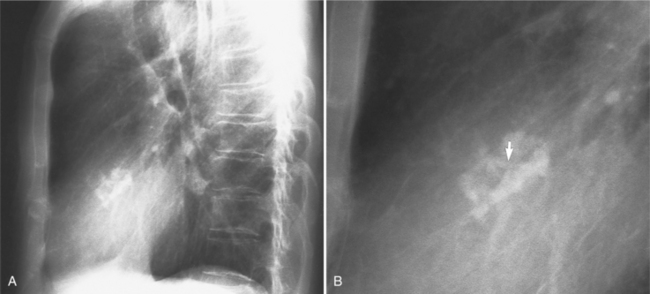
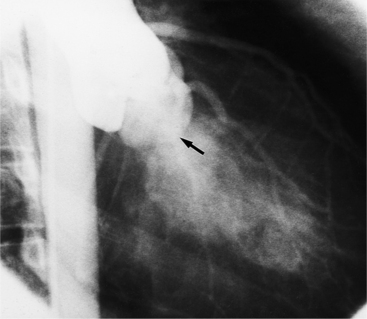
Distinctive calcifications on the lateral chest film characterize the congenital bicuspid aortic valve. Calcium is visible in the raphe of the valve and in the line of insertion of the shallow conjoint leaflet and the convex nonfused leaflet (Fig. 6-6). An acquired cause of a bicuspid valve is fusion of the commissures of the aortic leaflets in rheumatic heart disease.
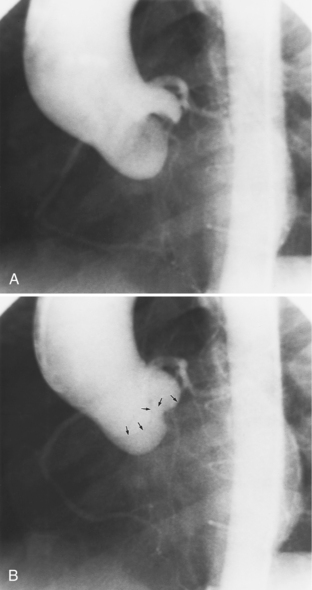
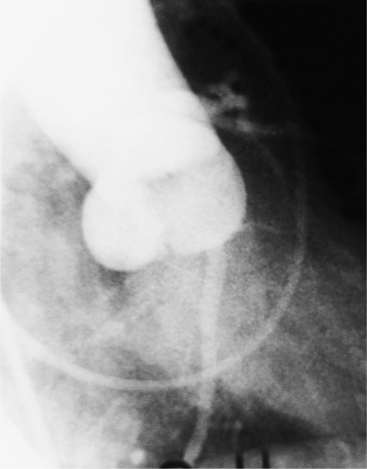
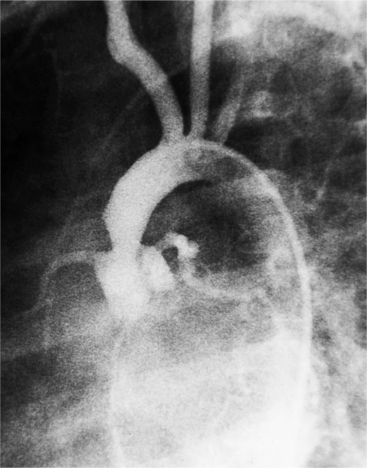
The angiographic appearance of a congenital bicuspid valve is two sinuses of Valsalva and two leaflets (Fig. 6-7). The valve orifice may be oriented in either an anteroposterior direction, which divides the leaflets into right and left cusps, or in a right-left direction, dividing the valve into anterior and posterior leaflets. If the cusps are on the right and left, there may be a raphe in the right cusp, and each sinus of Valsalva gives origin to a coronary artery. If the cusps are anterior and posterior, a raphe, if present, occurs in the anterior cusp, and both the coronary arteries arise from the anterior sinus of Valsalva. The normal aortic valve may acquire a bicuspid appearance when one commissure fuses as a result of rheumatic heart disease. In general, the congenital bicuspid valve will have one of the sinuses of Valsalva and its leaflet occupying half the circumference of the aortic annulus, whereas the acquired bicuspid valve with one fused commissure still demonstrates three sinuses of Valsalva of nearly equal size. In diastole, the congenital bicuspid valve may appear to have three sinuses of Valsalva because the raphe is visible and divides one of the two leaflets in half.
The congenital bicuspid aortic valve is recognizable most accurately during systole. The systolic appearance of a noncalcified congenital bicuspid valve typically includes domed and thickened leaflets (Fig. 6-8). The jet through the valve is usually slightly eccentric in direction but appears through the central part of the aortic annulus. In contrast, the jet through the rare unicommissural valve is quite eccentric.
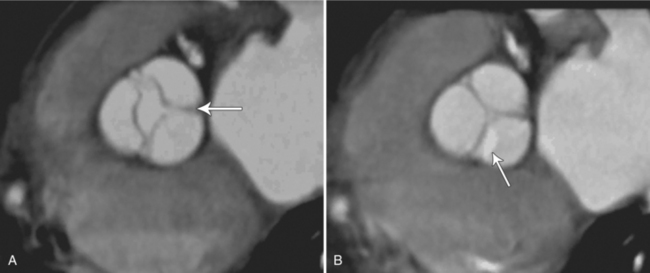
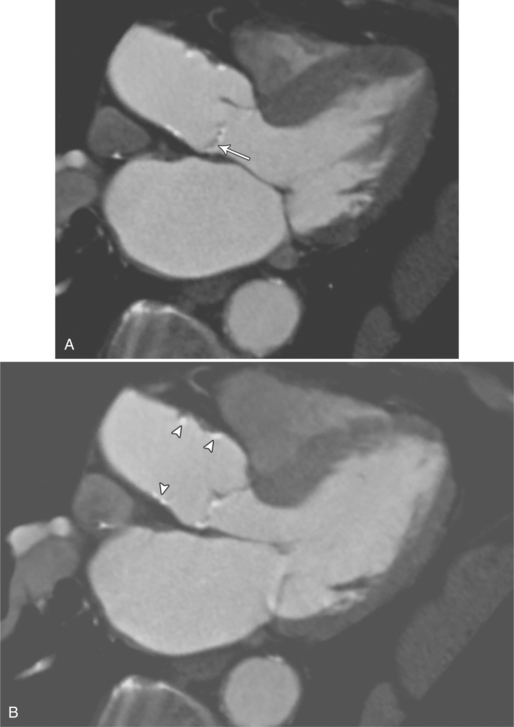
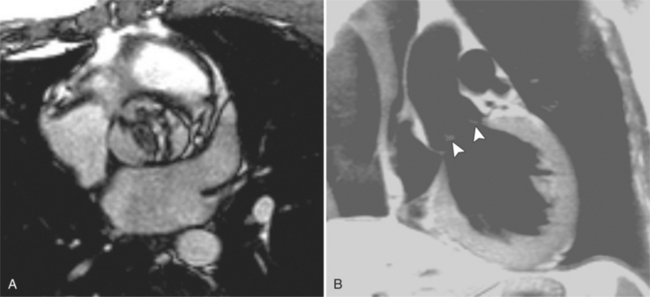
MDCT can be quite useful at characterizing congenital bicuspid aortic valves. Fig. 6-9 depicts a congenital bicuspid aortic valve in systole on MDCT in cross section and long axis, respectively. This patient has a normal systolic opening area with classic systolic dooming. Fig. 6-10 is a different case of a bicuspid aortic valve on MDCT. This valve demonstrates calcification of the cusps and diastolic prolapse. Cardiac MRI is more limited at depicting aortic valve leaflet morphology because the associated leaflet calcium causes profound signal void on MRI gradient pulse sequences, such as in Fig. 6-11. Black blood MRI pulse sequences may overcome this artifact.
Bicuspid aortic valves are associated with many types of congenital heart disease. Half of those with coarctation of the aorta have a bicuspid aortic valve (Fig. 6-12). More generally, any malformation of the aorta, such as aortic arch atresia or interruption or the hypoplastic left heart syndrome, has a high incidence of bicuspid aortic valve.
The congenital unicuspid aortic valve is intrinsically stenotic and may at times be incompetent. This valve may exist with no lateral attachments and appear as a diaphragm with a central opening. A second type of unicuspid valve has one lateral attachment to the aortic annulus with the commissure appearing as a raphe between the central point and the aortic wall. Both types of valve are rare. In systole, there is an eccentric jet against the posterior wall of the aorta with no leaflet tissue posteriorly. In diastole, a sinus of Valsalva may appear anteriorly but not posteriorly.
Acquired Valvular Aortic Stenosis
The aortic valve that is not stenotic at birth may become so in two ways:
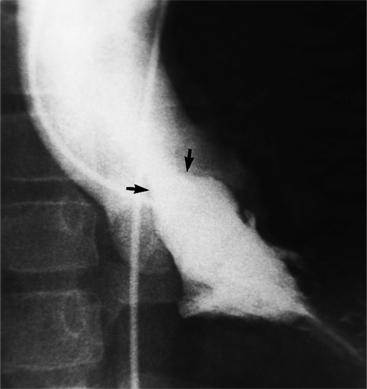
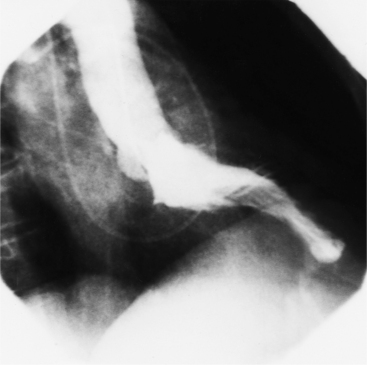
The end stage of any of these processes is a severely calcified valve, and it may be impossible to determine the initial process. In the extreme, the leaflets may be so thickened as to be akinetic. Unlike the congenital, noncalcified bicuspid aortic valve, these end-stage valves usually do not have a jet through them (Figure 6-13). The stream of contrast medium or blood through the distorted and curved linear orifice is turbulent. Doming of the leaflets is always seen in the noncalcified congenital aortic stenosis but is an inconstant feature in an end-stage aortic valve.
Calcific aortic stenosis in the adult results from rheumatic heart disease, occurs on a congenital bicuspid aortic valve, and occurs in the elderly. Although some overlap occurs, you can differentiate these three conditions by age and by the presence of other valvular lesions. The congenital bicuspid aortic valve begins to calcify in the fourth decade, whereas degenerative aortic stenosis affects those over 65 years of age. In developing countries, calcification in rheumatic aortic stenosis may appear in the late teens, but it is unusual in the United States until the fourth decade. With the aortic stenosis of rheumatic heart disease, mitral stenosis does not occur for at least 7 to 10 years after an episode of acute rheumatic fever, and aortic stenosis may develop about 7 years after that.
An important clue to the diagnosis of rheumatic disease in the aortic valve is the presence of mitral stenosis or regurgitation and calcification or thickening in the mitral leaflets in an otherwise functional valve. One characteristic of rheumatic aortic stenosis is the fusion of the commissures adjacent to the aortic wall. In general, these valves have either the usual appearance of a tricuspid valve or have one distorted sinus of Valsalva that occupies more than half the aortic annular circumference. When there is fusion of multiple commissures, the leaflets are usually so thickened and distorted that valve morphology is indistinguishable from other types of aortic stenosis.
Aortic stenosis in the elderly results from degeneration of the valve leaflets with subsequent thickening and calcification. Some patients have coronary calcification and calcification in the mitral annulus and in the aortic arch. These aortic valves are tricuspid and have clumps of calcium within the webs of the leaflets. In contrast to rheumatic valves, the commissures are not fused (Fig. 6-14).
MDCT can image calcific degenerative aortic valve stenosis (Figures 6-15, (6-16). The valve area can be planimetered from the short-axis views.
SUBVALVULAR AORTIC STENOSIS
Pathologic Abnormalities
Subvalvular aortic stenosis, or subaortic stenosis, consists of a heterogeneous group of abnormalities, several of which are associated with other types of cardiac malformation. These obstructions include:
Angiographic Findings
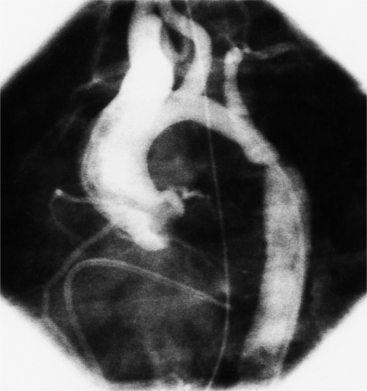
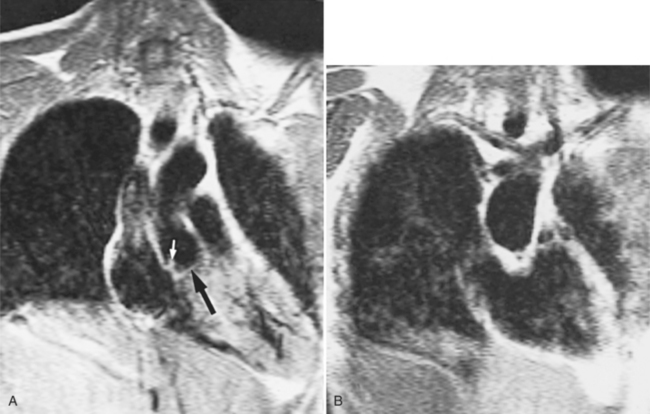
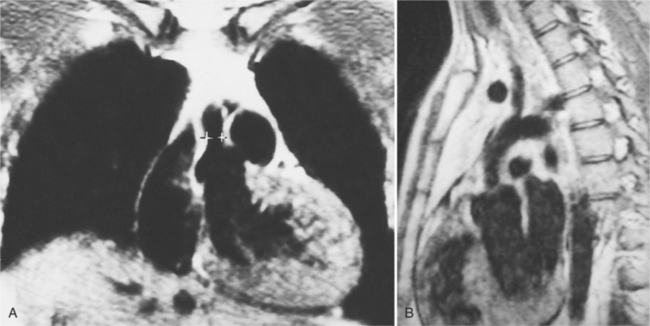
Optimal visualization of the angiographic features of most of these conditions is possible with biplane left ventriculography with cranial angulation in the left oblique projection. Because several of these malformations may be quite subtle (particularly discrete membranous subaortic stenosis), standard ventriculography may not demonstrate a lesion that is quite evident on pressure tracings. In these instances, an aortic root injection is useful because many of these valves are incompetent and the regurgitant stream outlines the subaortic chamber (Fig. 6-17). A magnetic resonance scan in planes aligned to the cardiac axis can sort out subaortic complexities with tomographic imaging (Fig. 6-18).
About 15% of patients with congenital obstruction to left ventricular outflow have discrete membranous subaortic stenosis. This consists of a 1- to 4-mm-thick membrane below the aortic valve. The membrane varies in position from just below the aortic valve to about 4 cm beneath it. The membrane may attach to the anterior leaflet of the mitral valve, and strands from this membrane may extend to the aortic cusps (Fig. 6-19). About one third to half of these patients have aortic insufficiency, and fewer than 5% have mild mitral insufficiency.
In tunnel subaortic stenosis, the obstruction extends 1 to 3 cm below the aortic annulus. One type is a fibromuscular bar extending near or on to the anterior leaflet of the mitral valve. Another type has a long conical narrowing in the subaortic region with little change between systole and diastole (Fig. 6-20). The left ventricle shows the usual signs of hypertrophy, but occasionally this overgrowth of muscle may produce bizarre shapes.
Idiopathic hypertrophic subaortic stenosis (discussed in Chapter 9) is a dynamic form of subaortic stenosis. The obstruction occurs in systole with the abnormal anterior systolic motion of the anterior leaflet of the mitral valve. During diastole, the stenosis disappears as the mitral leaflet resumes its normal position. A similar dynamic subvalvular stenosis may be seen in transposition of the great arteries. Here the mitral leaflet creates a subpulmonary stenosis as the left ventricle is connected to the pulmonary artery.
Subaortic Obstruction
Subaortic obstruction may rarely result from valve abnormalities or malalignment defects in complex congenital heart disease. Accessory mitral valve tissue, anomalous attachment of the mitral valve and its chordae tendineae, or a displaced annular insertion of the anterior leaflet result in outflow obstruction. The angiographic findings with each of these abnormalities varies; however, an asymmetric filling defect that moves into the subaortic region during systole and returns to a more posterior and inferior location during diastole is often visible during left ventriculography. Other complex congenital malformations have subaortic obstruction with conoventricular malalignment (e.g., aortic atresia and ventricular septal defect and in transposition with the aorta originating above an infundibular chamber).
SUPRAVALVULAR AORTIC STENOSIS
Classifications
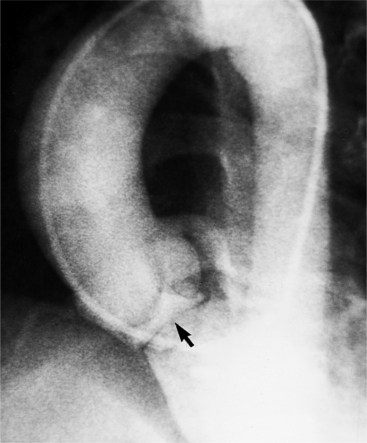
There are three types of supravalvular stenosis. The hourglass type consists of a narrow segment of the ascending aorta just distal to the origin of the coronary arteries (Fig. 6-21). The second type is a membrane or fibrous diaphragm at the sinotubular aortic junction. The least common is hypoplasia of the entire ascending aorta from the sinotubular junction to the origin of the brachiocephalic artery (Figure 6-22). In actual practice, these three forms overlap and are alternatively classified as discrete or diffuse stenoses, categories that correspond to the alternatives in surgical treatment.
AORTIC REGURGITATION
Aortic regurgitation results from a cusp abnormality, distortion of the aortic root, or dilatation of the aorta. Aortic valves that have stenosis generally also have some regurgitation. When the leaflets are abnormal, the regurgitation frequently is from rheumatic disease, infective endocarditis, or a bicuspid valve. When the ascending aorta is the cause of regurgitation, common causes are Marfan syndrome, aortic dissection, aortitis, ankylosing spondylitis, systemic hypertension, and syphilis (Box 6-2).
Chest Film Findings
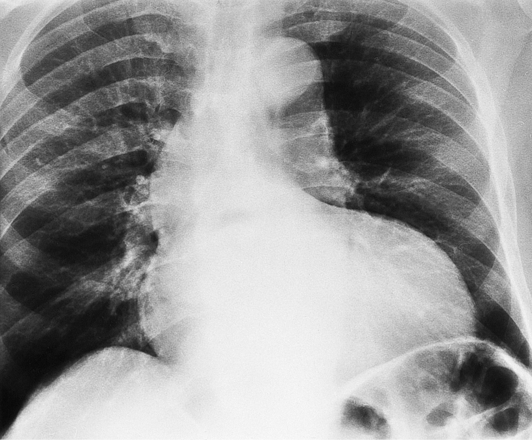
If the aortic regurgitation is both chronic and severe, the chest film hallmarks are left ventricular enlargement and dilatation of the entire aorta (Fig. 6-23). This pattern follows the principle that regurgitation of any of the heart valves enlarges structures on both sides of the insufficient valve. If the regurgitation is acute, signs of left ventricular failure are present—pulmonary edema and pleural effusions. Then, after several days, the left ventricle is visibly dilated.
Angiographic Technique
The typical examination is a biplane supravalvular aortogram performed in both oblique projections. The injection rate varies, depending on patient age, the size of the ascending aorta, and the clinical judgment of the severity of the runoff. For example, in the adult with suspected severe aortic regurgitation, an injection rate of 35 to 40 ml/sec over 2 seconds gives good visualization. Analysis of cuspal motion necessitates the cine technique.
Magnetic Resonance Imaging Technique
Cine MRI and color flow Doppler ultrasound can identify and quantify valvular regurgitation. On both techniques, the imaging plane is rotated to include the aortic root and the regurgitant jet. Cine MRI (gradient-refocused MRI), a “white blood” technique, is set up to provide repetitive loops of a cardiac cycle with both magnitude and phase reconstruction. Aortic regurgitation is identified as a discrete area of signal loss coming from the aortic valve into the left ventricle during diastole (Figure 6-24).
Cardiac-Gated Multidetector Computed Tomography
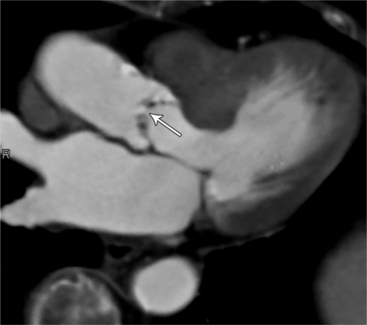
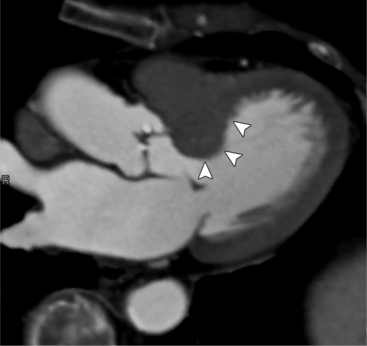
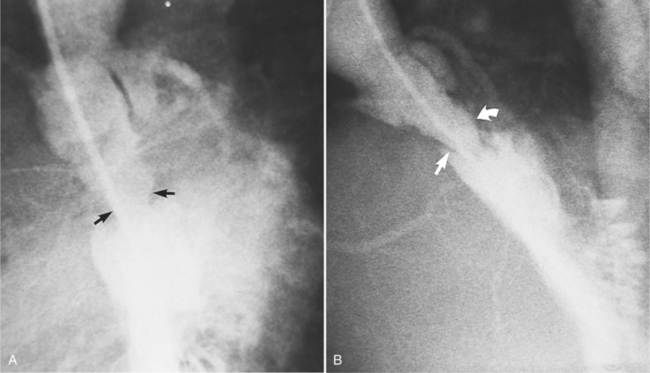
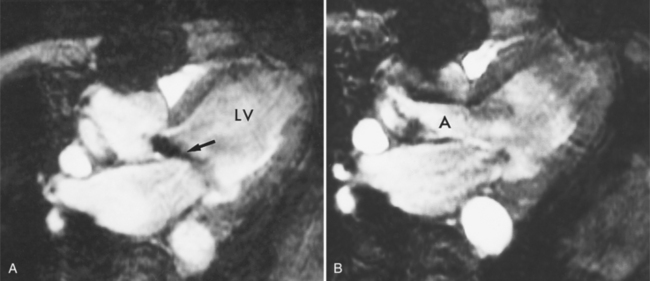
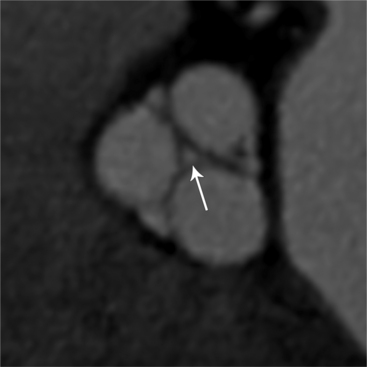
MDCT can detect the presence of aortic regurgitation by demonstrating in diastole the leaflet malcoaptation and the resultant regurgitant orifice. MDCT can demonstrate that the planimetered area of the central valvular leakage area correlates well to echocardiogram or MRI grades of aortic regurgitation severity. As with cases of aortic stenosis, MDCT has the added advantage of assessing for concurrent aortopathies. (Figures 6-25 and (6-26 demonstrate cases of mild and severe aortic regurgitation, respectively.
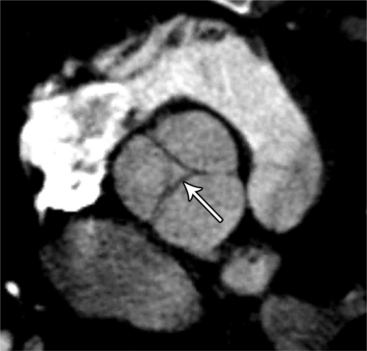
FIGURE 6-26 Severe aortic regurgitation. The aortic valve has a large central regurgitant area (arrow).
Specific Causes of Aortic Regurgitation
Fibrosis from the valvulitis in rheumatic heart disease is the most common aortic valve abnormality that causes regurgitation. These valves have thickened cusps that are shortened by the fibrotic process and may have some commissural fusion. Depending on the severity of the rheumatic process, the leaflets may have no visible calcium or, conversely, they may be reduced to irregular lumps with poor motion. The regurgitant jet is usually central unless the valve commissures are fused asymmetrically. In degenerative aortic valve disease, which occurs in persons over age 65, the cusps are thickened and immobile but without commissural fusion. Then regurgitation occurs over a broad front that is essentially the same diameter as the aortic annulus (Fig. 6-27
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree