Atherosclerosis
60–90 %
Age >50
Common in male
Fibromuscular dysplasia (FMD)
10–30 %
Age 20–50
Common in female
Others (rare)
<10 %
Common in female
Vasculitis
Takayasu’s arteritis
Polyarteritis nodosa (PAN)
Buerger’s disease
Dissection (renal arterial or aortic)
Neurofibromatosis
Midaortic syndrome
Radiation injury
Extrinsic compression (tumor, hematoma)
There are about two to four million individuals with RAS in the United States alone. Patients with atherosclerotic RAS are usually over 60 years of age and more likely to be male.
Incidence of RAS resulting in hypertension is about 1–5 % among all patients with hypertension.
The clinical presentation of patients, clinical signs, and risk factors are summarized in Table 5.2.
Table 5.2
Clinical signs of hypertension secondary to RASa
Sudden onset of severe hypertension |
Accelerated or malignant hypertension |
Onset of hypertension <30 or >60 years of age |
Hypertension with rapidly progressive renal failure |
Hypertension unresponsive to appropriate three-drug therapy |
Renal failure in response to ACEb inhibitor |
Hypertension associated with an upper abdominal bruit over kidney |
Episodes of recurrent severe hypertension and pulmonary edema |
Percutaneous endovascular or surgical revascularization may significantly improve hypertension and even improve renal function in some patients who have atherosclerotic RAS.
Imaging
Plain Film Radiography
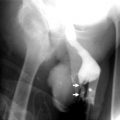
Plain film radiography may demonstrate calcification of renal arteries in patients with hypertension (Fig. 5.1).
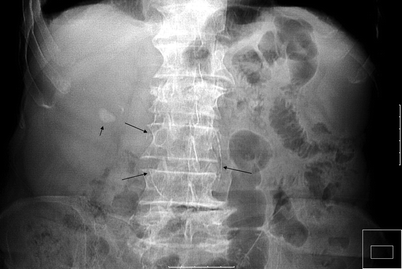
Fig. 5.1
Abdominal aortic aneurysm with RAS. Abdominal radiography shows right renal stone (short arrow) and calcification of abdominal aortic aneurysm (long arrows) of a patient with hypertension and left upper abdominal bruit over the level of renal artery
Intravenous Pyelography
Intravenous pyelography (IVP) is mentioned as a test of historical significance for the diagnosis of RAS.
Major findings on IVP that suggest the presence of unilateral ischemia include decreased renal size and delayed renal parenchymal contrast enhancement with delayed caliceal appearance time when compared to the contralateral kidney.
IVP is significantly less sensitive than other imaging examinations, and a negative test result cannot exclude RAS reliably. Furthermore, bilateral disease can be missed.
Ultrasonography
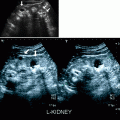
Gray-scale US finding of narrowing of renal arteries at the ostium with or without calcified plaque is suggestive of atherosclerotic stenosis.
On color flow Doppler, focal color aliasing with mosaic pattern, poststenotic turbulence, and soft tissue color bruit suggests an underlying stenosis with increased peak systolic velocity (PSV), and poststenotic dilatation of the renal artery may also be typically observed (Fig. 5.2).
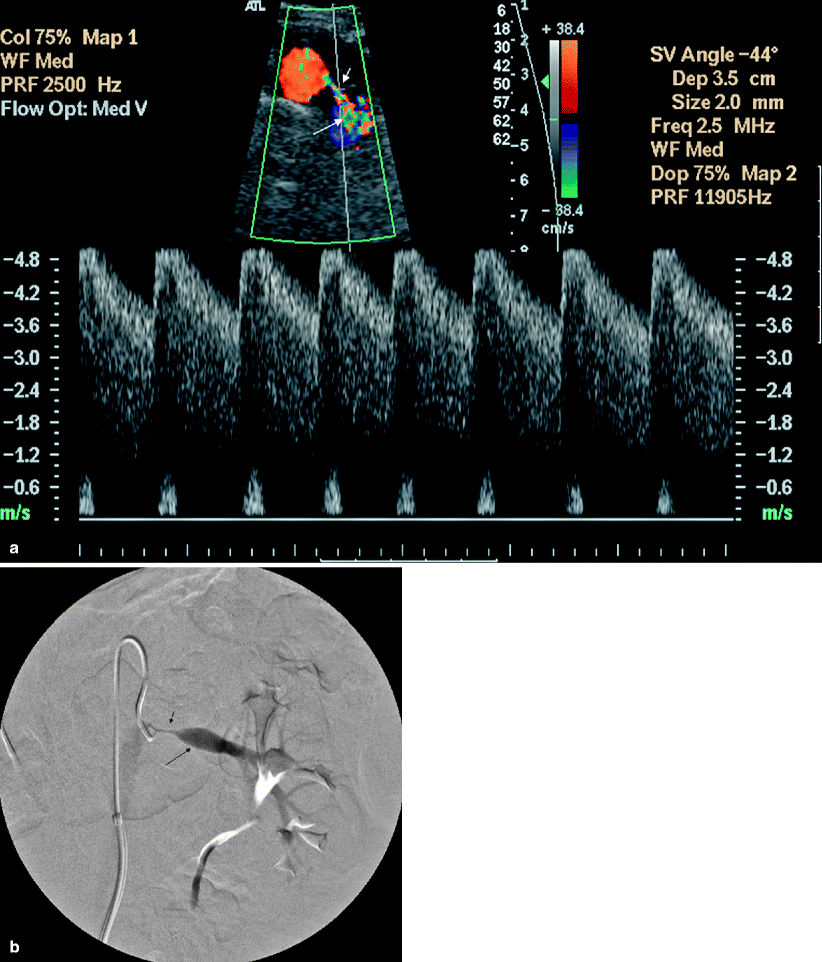
Fig. 5.2
Renal artery stenosis. (a) Transverse color flow Doppler US demonstrates focal aliasing with turbulent flow (short arrow) near the origin of the left main renal artery from the aorta. On spectral Doppler in the region of color aliasing, the PSV measures more than 4.8 m/s, consistent with RAS. Poststenotic dilatation of the renal artery can also be observed (long arrow). (b) Preprocedural DSA image shows the high-grade stenosis (short arrow) with poststenotic dilatation (long arrow) and confirms the diagnosis
Peak systolic velocity focally increases at the anatomic site of a hemodynamically significant stenosis in the main renal artery. PSV in the main renal artery is greater than 180 or 200 cm/s (Fig. 5.3).
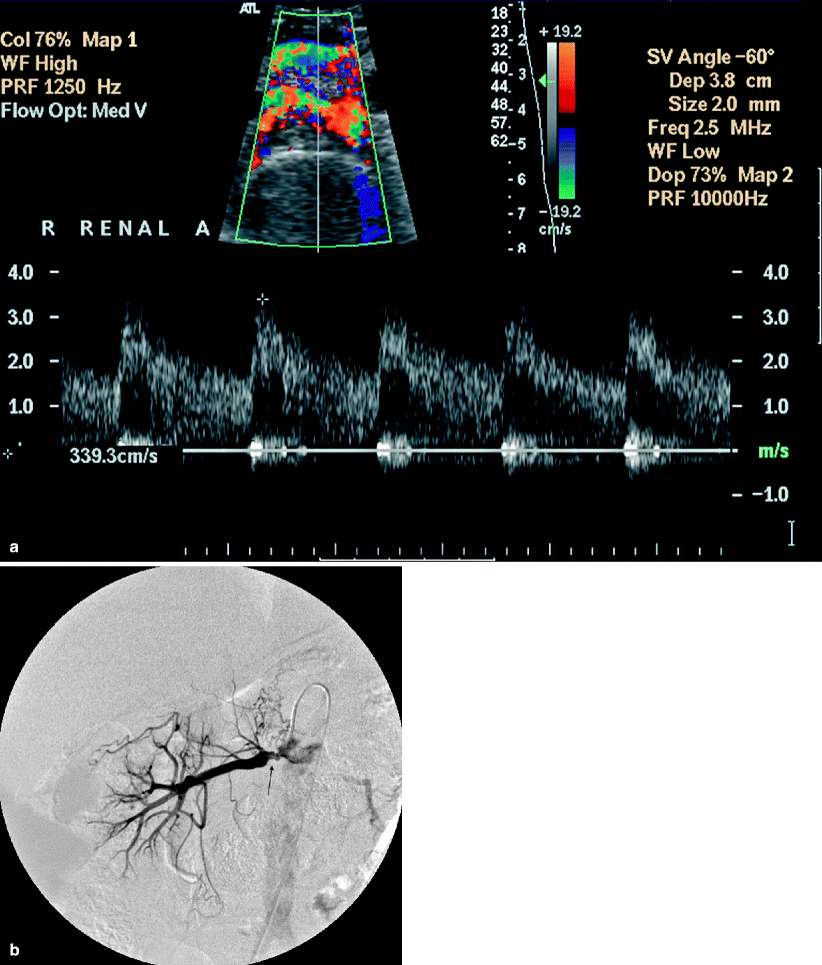
Fig. 5.3
Renal artery stenosis. (a) Transverse color flow Doppler US demonstrates focal aliasing with turbulent flow and soft tissue vibration artifacts near the origin of the right main renal artery from the aorta. On spectral Doppler in the region of color aliasing, the PSV measures 339 cm/s, consistent with RAS; aortic peak systolic velocity is 84 cm/s at this level (not shown). (b) Arterial DSA image before intervention shows the irregular atherosclerotic plaques with stenosis (arrow) and confirms the diagnosis
Reno-aortic ratio (RAR) can be obtained by dividing the PSV at the origin of the main renal artery with PSV of the aorta. This ratio compensates individual variations in cardiac output and a reno-aortic ratio (RAR) greater than 3.0–3.5 to be diagnostic of hemodynamically significant stenosis of more than 50 %.
The deep location of the main renal arteries may limit direct visualization of RAS on color flow Doppler, but evaluating parenchymal arterial waveform abnormalities provides an indirect method of diagnosing RAS.
The spectral Doppler appearance of normal native renal arterial flow should include rapid systolic upstroke to an early systolic peak with gentle decrease in flow velocity during late systole and diastole. RAS may result in loss of this characteristic spectral pattern.
Pulsus tardus et parvus pattern (rounding and flattening of the systolic peak) may be observed distal to RAS (Fig. 5.4).
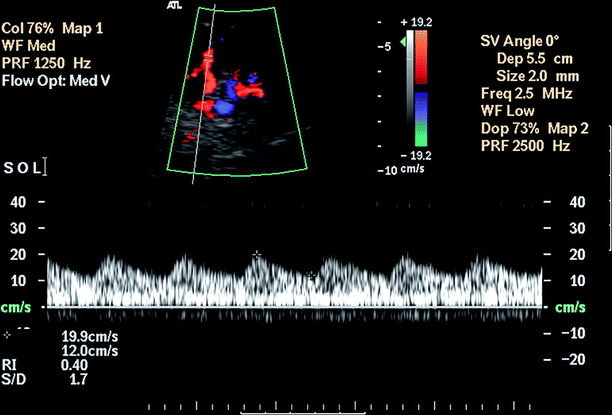
Fig. 5.4
Renal artery stenosis. Pulsus tardus et parvus waveform with rounding and flattening of the systolic peak with delayed upstroke indicates a high-grade proximal stenosis
An “acceleration time” (AT) of greater than or equal to 0.07 s has high accuracy, sensitivity, and specificity for detecting RAS (Fig. 5.5).
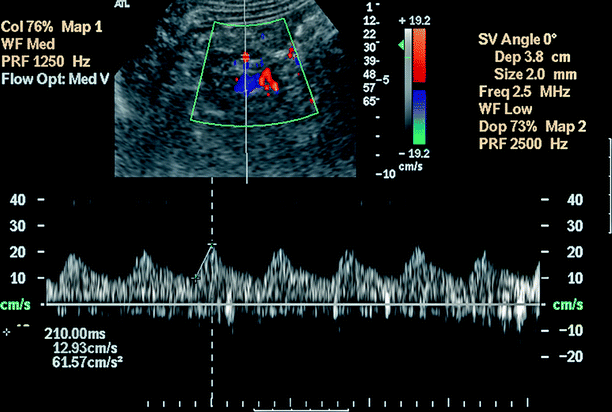
Fig. 5.5
Renal artery stenosis. Acceleration index and acceleration time: Acceleration index is the slope of the line connecting the point of onset of systole and the early systolic peak. Acceleration time (AT) is the length of time between these two points. Abnormal waveform with a delay in systolic acceleration indicates a proximal stenosis of the renal artery. Pulsus tardus et parvus waveform with a slowly accelerating slope and markedly prolonged AT (210 ms) indicating a high-grade proximal stenosis
Pre-administration of 25 mg of captopril 1 h before Doppler ultrasound examination exaggerates the “pulsus tardus et parvus” waveform in the parenchymal vessels of patients who have RAS.
The segmental artery resistive index (RI) which is calculated as [PSV − end diastolic velocity]/PSV may be useful in detecting concomitant renal disease. The normal RI in adults should be less than 0.70 in native renal artery.
Resistive index is an independent predictor of cardiovascular target organ damage and renal disease. In chronic renal disease, elevated RI has been shown to be an independent risk factor of worsening renal function and mortality.
The RI represents more than basic resistance, and it may be affected by many factors such as heart rate, valsalva by the patient, and arterial compliance.
A difference in RI of both kidneys (>0.07) may suggest RAS.
Some investigators also use ratio of PSV in the main renal artery to the PSV in the interlobar arteries (RIR), and threshold values of RIR greater than 5 with PSV less than 15 cm/s in the interlobar artery were found to give high sensitivity (91 %) and specificity (87 %) for RAS.
Criteria for hemodynamically significant renal artery stenosis in native vessels (>60 %) stenosis are summarized on Table 5.3.
Table 5.3
Criteria for hemodynamically significant renal artery stenosis in native vessels (>60 % stenosis)
Direct criteria
Peak systolic velocity (PSV)
>180 cm/s in main renal artery
Renal artery-aortic ratio (RAR)
>3.0–3.5
Resistive index (RI)
>0.7
Indirect criteria
Renal artery interlobar artery ratio (RIR)
>5
Acceleration time (AT)
>0.07 s
Acceleration index (AI)
>3 m/s2
Parvus tardus waveform pattern in intrarenal vessels
Computed Tomography
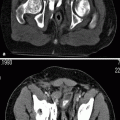
Multidetector computed tomography (MDCT) angiography is comparable to DSA in the detection of hemodynamically significant RAS with a sensitivity and specificity of up to 96 and 99 %, respectively (Figs. 5.6 and 5.7).
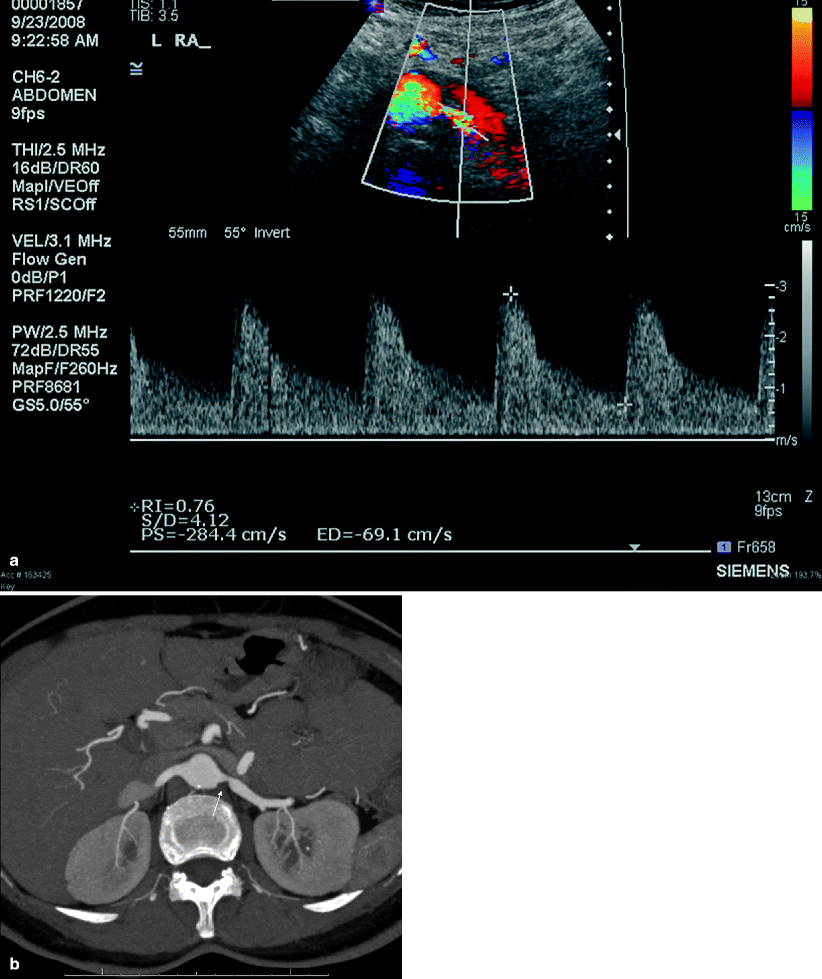
Fig. 5.6
Renal artery stenosis. (a) Transverse color flow Doppler US demonstrates focal mosaic pattern with turbulent flow near the origin of the left main renal artery. In the region of suspected stenosis, the PSV measures more than 284 cm/s, suggesting moderate stenosis. (b) Axial MIP MDCT angiography image shows 60 % stenosis (arrow) with poststenotic dilatation
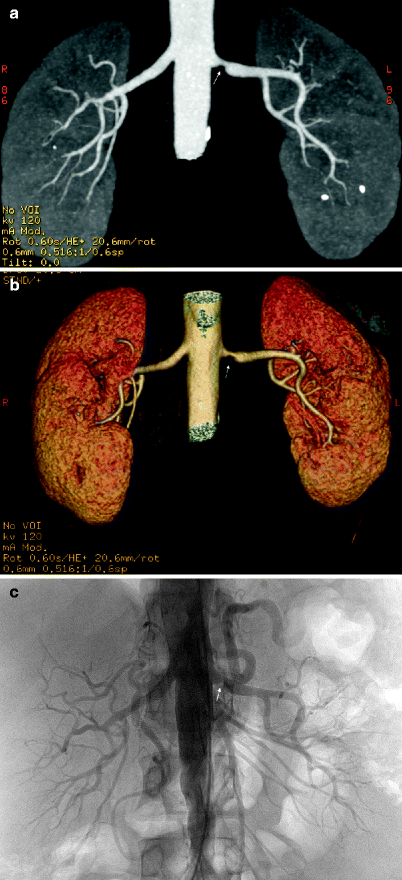
Fig. 5.7
Renal artery stenosis. Coronal MIP (a) and volume rendering (b) images reveal a stenosis at the origin of the left renal artery (arrow). Note aortic wall calcifications and renal stones at the lower pole of the left kidney. (c) Diagnostic angiography before intervention confirms hemodynamically significant RAS (arrow)
Secondary signs of RAS, such as poststenotic dilatation, decrease in renal size, cortical thinning, and decreased cortical enhancement, are also helpful in recognizing RAS on CT imaging.
Dynamic contrast-enhanced MDCT can show delayed nephrographic progression in the affected kidney.
Combined evaluation of source images, multiplanar reconstruction, shaded surface display, maximum intensity projection, and volume rendering is usually necessary to obtain good results (Fig. 5.7).
Up to 30 % of patients have accessory renal arteries, and CT angiography has been shown to adequately identify these arteries.
Selective DSA with pressure gradient measurement is necessary to assess the physiologic significance of RAS.
Magnetic Resonance Imaging
Renal MR angiography allows accurate anatomic examination of the main renal arteries in patients suspected of having RAS (Fig. 5.8).
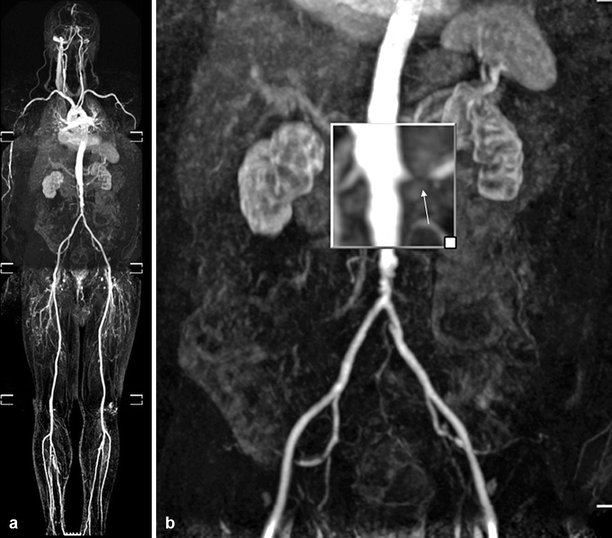
Fig. 5.8
Atherosclerotic RAS. Coronal whole-body MR angiography image shows atherosclerotic stenosis of left renal artery (a). Significant stenosis of the left renal artery (arrow) with renal cortical atrophy is seen on magnified image (b)
Many investigators currently advocate using contrast-enhanced MR angiography as the primary noninvasive screening modality for RAS.
Different studies have reported excellent correlation between DSA and MR angiography for detection of hemodynamically significant atherosclerotic RAS, with sensitivities and specificities up to 100 and 90 %, respectively (Fig. 5.9).
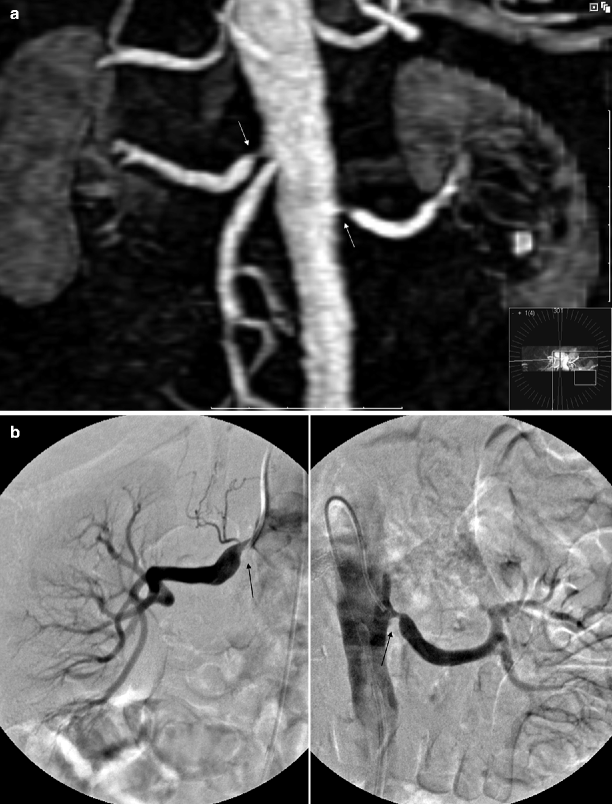
Fig. 5.9
Atherosclerotic RAS. (a) Coronal 3D gadolinium-enhanced MR angiography image shows bilateral atherosclerotic renal artery stenosis (arrows). (b) Selective renal DSA images obtained before endovascular intervention show bilateral high-grade stenoses (arrows) with poststenotic dilatation and confirm the diagnosis
Nuclear Scintigraphy
Angiotensin-converting enzyme (ACE) inhibitor scintigraphy of a kidney with RAS may exhibit impaired function during ACE inhibition.
A decline in the glomerular filtration rate (GFR) of the affected kidney can be induced by ACE inhibition in patients who have unilateral RAS.
ACE inhibitor induces significant change in the time activity curves of the affected kidney when compared either with base scintigraphy before ACE ingestion or with the contralateral normal kidney.
Specifically, ACE inhibitors induce renal retention of the radiopharmaceutical in patients who have renovascular hypertension because of decreased urinary output secondary to reduced GFR.
Although captopril renal scintigraphy provides physiologic information, it does not provide anatomic confirmation of abnormal physiology and is limited in evaluating patients who have bilateral RAS.
The most common radiopharmaceuticals are Tc-99m mercaptoacetyltriglycine (MAG3 scan) or Tc-99m diethylenetriaminepentaacetic acid (DTPA scan).
Technetium-99m DTPA and Tc-99m MAG 3 have biological half-life as 6 and 2.5 h, respectively.
Pathology
Atherosclerosis-related renal artery stenosis results from essential hypertension (benign nephrosclerosis), remote infarcts from aortic atheroemboli, or both.
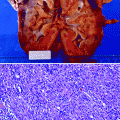
In benign nephrosclerosis, the kidneys are symmetrically small, with granular subcapsular surfaces and thin cortices, the extent of which depends on the severity of the disease. The intima of the renal artery is eccentrically thickened, as the media acquires myointimal cells that deposit connective tissue components. Foamy histiocytes (lipid) are trapped, and fibrosis develops. The internal elastic lamina is not preserved in these areas. Cholesterol clefts and calcification indicate long-standing disease (Figs. 5.10 and 5.11).
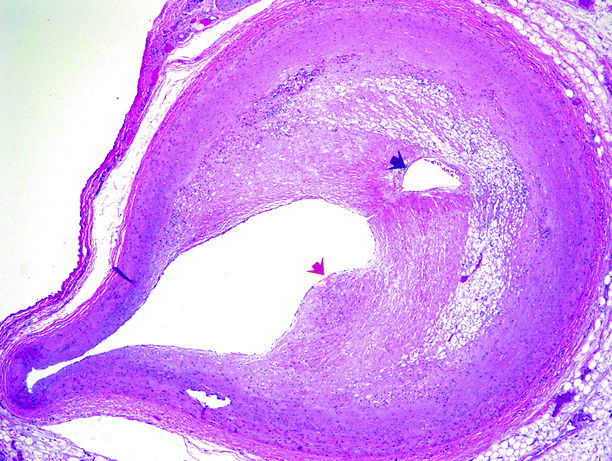
Fig. 5.10
Muscular artery involved by an atheromatous plaque. A fibrous cap (red arrow) overlies the core. Neovascularization appears to have produced a secondary lumen (black arrow)
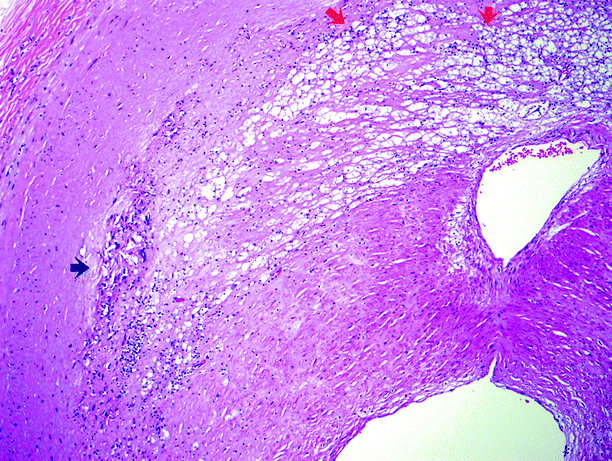
Fig. 5.11
Higher power view of an atheromatous plaque. Cholesterol crystalline aggregates are indicated by the black arrow; the small dark cells in that area are lymphocytes. The red arrows indicate more of the core, consisting of lipids, cellular debris, fibrin, and abundant pale-staining macrophages
Renal artery stenosis may also rarely be the result of intrinsic or extrinsic neoplasms (Figs. 5.12 and 5.13).
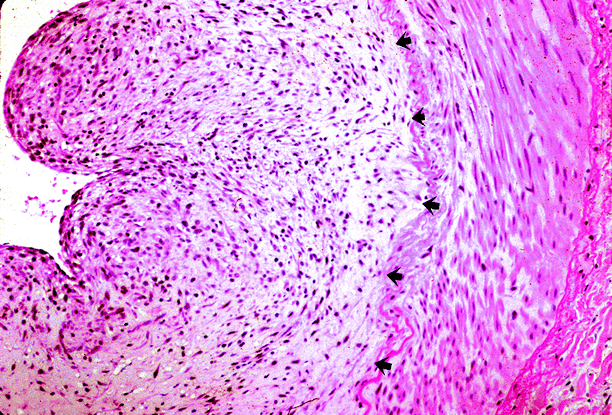
Fig. 5.12
Renal artery stenosis. Patient was a hypertensive adult with neurofibromatosis, who was found to have a stenotic renal artery. The renal artery specimen shows diffuse intimal expansion by neoplastic neural tissue (arrows) (Case courtesy of Gretta Jacobs, M..D)
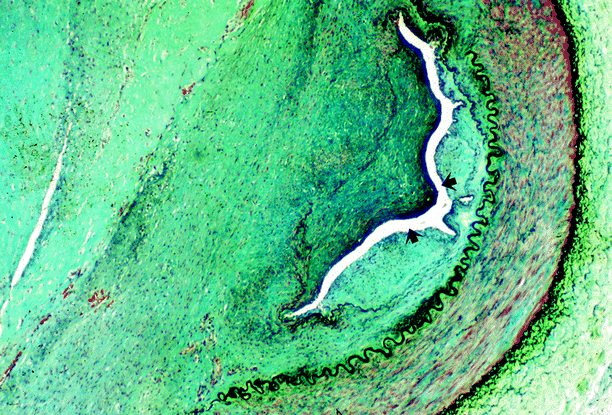
Fig. 5.13
Renal artery stenosis. Same case as shown in Fig. 5.12. The renal artery lumen (arrows) is almost entirely occluded by an expansile neural neoplasm (Case courtesy of Gretta Jacobs, M.D.)
Differential Diagnosis
The most common cause of RAA is atherosclerosis. Annular calcification occurs in more than 50 % of atherosclerotic RAAs; the differentiation between a calcified renal artery aneurysm and a renal calculus is especially important.
Abdominal bruit may be present in patients with RAS but can also be present in other conditions such as abdominal visceral arterial stenosis, arteriovenous fistula, or celiac artery compression syndrome.
Scintigraphic differential diagnosis of RAS includes acute tubular necrosis which can present with findings similar to those seen with renin-mediated hypertension due to RAS.
Patients who are poorly hydrated may demonstrate prolonged parenchymal retention with decreased excretion of tracer and hypotension following administration of ACE inhibitor resulting in a false-positive interpretation for renovascular hypertension.
Pearls and Pitfalls
Rich collateral blood flow around a high-grade stenosis or occlusion may obscure the diagnosis of RAS.
Systemic factors, such as age, hypertension, and diabetes, also affect vessel compliance, and peripheral vascular resistance may minimize development of the parenchymal “pulsus tardus et parvus” waveform distal to a stenosis.
Peak systolic velocity of >180 cm/s in renal artery is the most sensitive criteria to diagnose RAS.
In general, MR angiography tends to overestimate the degree of stenosis. When atherosclerotic lesions are calcified or eccentric, their severity tends to be underestimated with shaded surface display when compared with maximum intensity projection.
MR angiography cannot evaluate the residual lumen within a metallic stent, which can be done with CT angiography, DSA, or Doppler US (Fig. 5.14a).
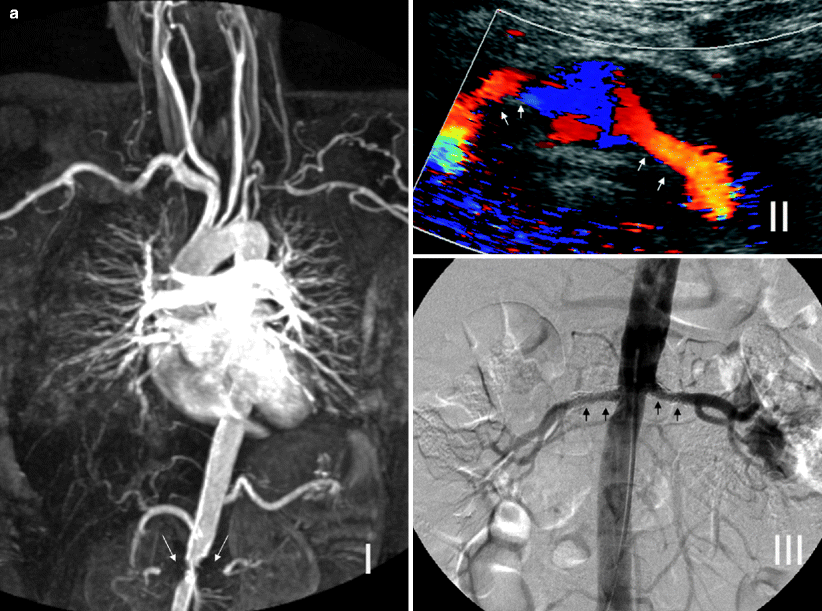
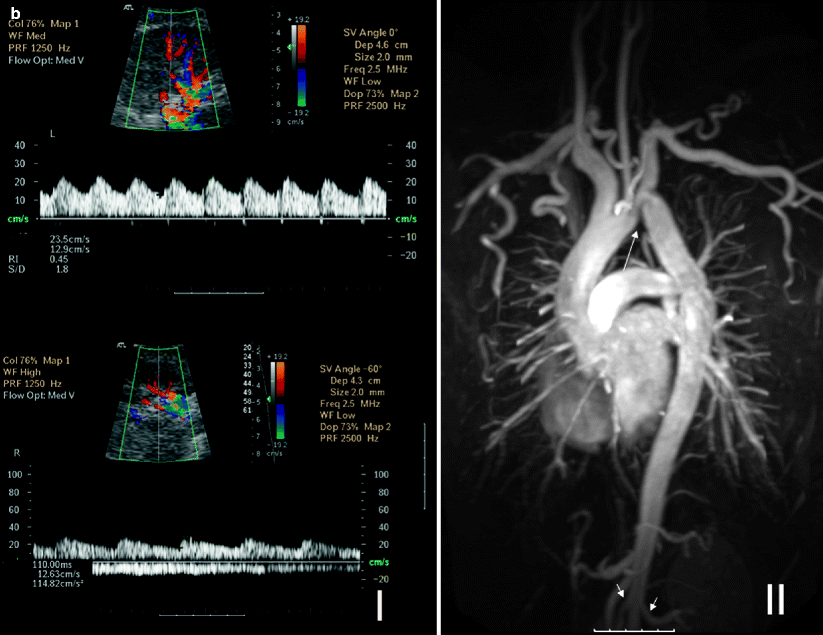
Fig. 5.14
(a) Bilateral renal artery stents for RAS secondary to Takayasu arteritis. MR angiography cannot evaluate the residual lumen within the metallic stents, and blooming artifact gives the impression of bilateral occluded renal arteries with significant aortic stenosis (long arrows) (I). Color Doppler ultrasound of the same patient shows patent renal stents, and DSA image confirms the patency (short arrows) (II and III). (b) Coarctation of aorta. Spectral Doppler studies reveal bilateral pulsus tardus et parvus waveforms indicating delay in systolic acceleration in the right and left parenchymal renal arteries. The AT on the right is 110 ms, and RI on the left is 0.45 (I). These findings would indicate the possibility of proximal RAS. Coronal 3D gadolinium-enhanced MR angiography image shows coarctation of the aorta just after the origin of the left subclavian artery (long arrow). Note prominent thoracic arterial collaterals and proximal parts of the patent renal arteries (short arrows) (II)
Other causes of secondary hypertension include renal disease, hyperaldosteronism, pheochromocytoma; Cushing’s syndrome needs laboratory findings or imaging studies for differential diagnosis where aortic coarctation, Takayasu arteritis, William’s syndrome, polyarteritis nodosa, and other rare causes of RAS (neurofibromatosis, renal aneurysms, renal arteriovenous fistula, radiation injury, and extrinsic compression) should also be kept in mind while evaluating vascular imaging examinations (Fig. 5.14b).
Fibromuscular Dysplasia (FMD)
General Information
Fibromuscular dysplasia tends to occur in young to middle aged women (<40 years) and is the most common cause of renovascular hypertension in children and young adults. Among adults, FMD is more common among women, with a prevalence eight times higher compared to men.
FMD is a correctable cause of RAS and has a propensity to occur in Afro-Americans.
The histopathological classification reflects different forms of the disease according to the primarily involved layers of arterial wall, intimal fibroplasia, medial fibromuscular dysplasia, and adventitial fibroplasia.
Medial fibromuscular dysplasia is the most common and has three subtypes: medial fibroplasia (common), medial hyperplasia, and perimedial dysplasia.
FMD may be bilateral in up to 50 % of cases and associated with the involvement of other aortic branches in 1–2 %.
Imaging
Ultrasonography
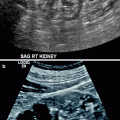
Fibromuscular dysplasia is often found in younger patients, with female predominance. Gray scale and color Doppler findings of increased PSV of >180 cm/s in the distal two-thirds of the renal artery are suggestive of FMD.
“String of beads” representing aneurysmal focal dilatation of the renal artery can also be seen with color flow Doppler (Fig. 5.15).
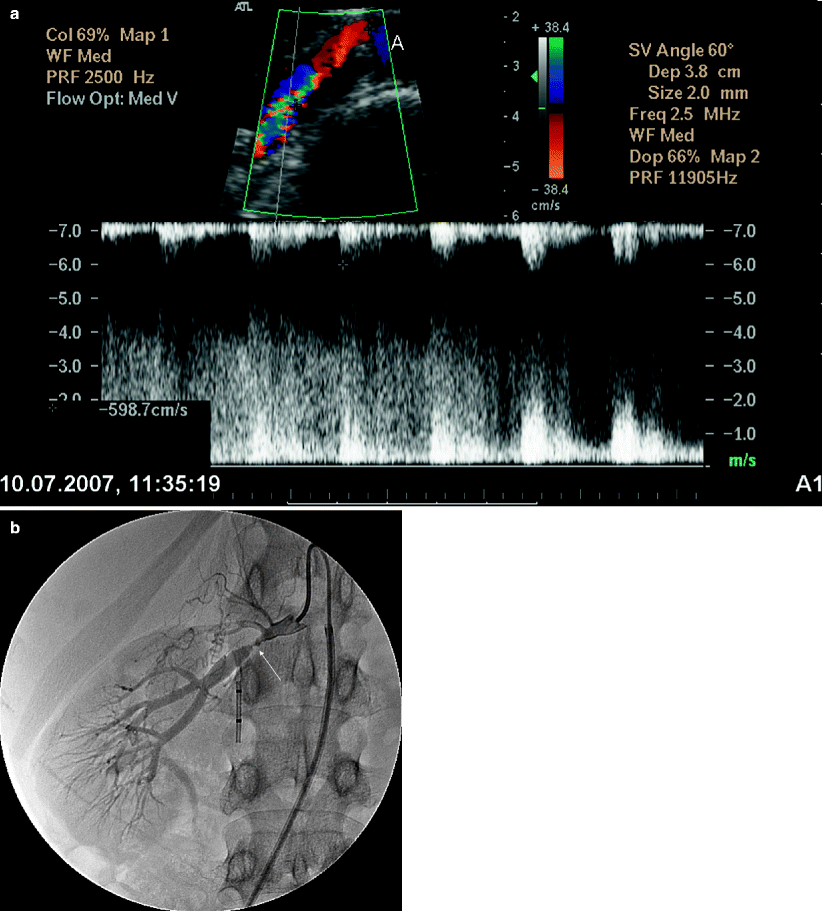
Fig. 5.15
Fibromuscular dysplasia causing RAS. (a) Oblique transverse color flow Doppler US demonstrates focal aliasing with turbulent flow at the distal part of the right main renal artery far from the aorta with a PSV of 598 cm/s. (b) Angiography image obtained before intervention shows high-grade stenosis (arrow) with beading appearance. Arterial collaterals are present proximal to the RAS
Computed Tomography
MDCT can demonstrate a string-of-beads appearance of renal artery.
Magnetic Resonance Imaging
MR angiography is an alternate imaging that can be used if MDCT is contraindicated in patients with iodine allergy (Fig. 5.16).
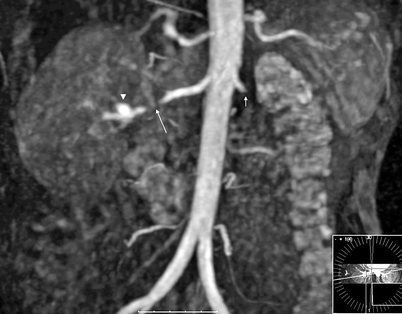
Fig. 5.16
Fibromuscular dysplasia causing RAS. Coronal 3D gadolinium-enhanced MR angiography image shows high-grade stenosis (near total occlusion) (long arrow) of the distal third of the right main renal artery with a distal aneurysm (arrowhead). Note previously occluded left renal artery (short arrow)
Digital Subtraction Angiography
Angiography plays the pivotal role in the diagnosis as well as selection of the patients for treatment.
String-of-beads appearance of distal two-thirds of renal artery.
Pathology
Fibromuscular dysplasia is a group of histologically distinct lesions that have a similar clinical presentation. There are three general categories of fibromuscular dysplasia: intimal fibroplasia, medial fibroplasia, and periarterial fibroplasia.
Intimal fibroplasia is a circumferential intimal thickening in a renal artery segment with preservation of the internal elastic lamina.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree