Chapter Outline
Budd-Chiari Syndrome and Sinusoidal Obstruction Syndrome
Cavernous Transformation of the Portal Vein
Superior Mesenteric Vein Thrombosis
Splenic Vein and Inferior Mesenteric Vein Thrombosis
Hemolysis, Elevated Liver Enzymes, Low Platelets (HELLP) Syndrome
The liver has a unique, dual blood supply in which 25% of the flow comes from the hepatic artery and 75% through the portal vein ( Fig. 90-1 ). There is an inverse relationship between these two blood supplies. If portal flow decreases, arterial flow will increase as if an impedance has been removed. In addition, there are several communications between these vessels that open in response to nervous and humoral factors: trans-sinusoidal, transvasal, and transplexal. When vascular compromise develops, the volume and direction of blood flow of an individual vessel will be altered. Multidetector computed tomography (MDCT), Doppler ultrasound, and magnetic resonance imaging (MRI) are sensitive in the diagnosis of these perfusion disorders and are discussed in this chapter.







Transient Hepatic Attenuation Differences (THADs) and Transient Hepatic Intensity Differences (THIDs)
Intraparenchymal perfusion disorders such as transient hepatic attenuation differences (THADs) and transient hepatic intensity differences (THIDs) are epiphenomena of alterations of the dual vascular supply of the liver. There is a compensatory relationship between hepatic arterial and portal venous blood supply so that arterial flow increases when portal flow decreases ( Fig. 90-2 ). This is made possible by communication among the main vessels, sinusoids, and peribiliary venules that dilate in response to autonomic nervous system and humoral factors activated by hepatic demand for oxygen and metabolites. THADs and THIDs are areas of parenchymal enhancement visible during the hepatic arterial phase after the intravenous administration of contrast media. These lesions can be classified by morphology, etiology ( Figs. 90-3 to 90-6 ), and pathogenesis.






Thads and Thids Associated with a Mass Lesion
Benign and malignant masses produce two morphologic types of THADs and THIDs by four pathophysiologic mechanisms: directly, by siphoning effect of the mass (lobar multisegmental shape); indirectly, by means of portal hypoperfusion (sectorial shape) due to portal branch compression or infiltration; by thrombus, resulting in a portal branch blockade; or by flow diversion caused by an arterioportal shunt.
Lobar multisegmental THADs and THIDs occur when a benign hypervascular lesion or an abscess induces an increase in the primary arterial inflow, which leads to surrounding parenchyma perfusion, the so-called siphoning effect. These THADs and THIDs do not assume a triangular shape, but a straight border may be present between the arterial phenomena from adjacent parenchyma.
Sectorial THADs and THIDs follow hepatic vessel dichotomy and appear as triangular areas that result from the strict relationship between the portal hypoperfused area and the arterial reaction. These can be seen in benign and malignant tumors as well as in abscesses due to the spread of inflammatory mediators. The THADs and THIDs can be wedge or fan shaped.
Thads and Thids Not Associated with a Mass Lesion
THIDs and THADs can be seen in the absence of a focal lesion as a result of three mechanisms: portal hypoperfusion due to portal branch compression or thrombosis; flow diversion by arterioportal or an anomalous blood supply; or inflammation of the bile ducts or gallbladder.
Sectorial THIDs and THADs are usually caused by portal hypoperfusion due to portal vein or hepatic vein thrombosis, long-standing biliary obstruction, or arterioportal shunt that may be congenital, traumatic, or due to cirrhosis. These THIDs and THADs can have a globular shape, especially when they are adjacent to Glisson’s capsule.
Polymorphous THADs and THIDs have four major causes: external compression by a rib or subcapsular collection; anomalous blood supply from atypical arteries, collateral venous vessels, or accessory veins, especially in segment IV of the liver; inflammation of adjacent organs, such as cholecystitis and pancreatitis that spread inflammatory mediators and reduce portal inflow because of interstitial edema; and trauma, biopsy, or radiofrequency ablation of hepatic tumors.
In patients with obstruction of the superior vena cava, the medial segment of the left lobe (segment IV) of the liver will hyperenhance because of collateral veins. The internal mammary vein connects to the left portal vein by the paraumbilical vein.
Diffuse THADs and THIDs can be seen in right-sided heart failure (see later), Budd-Chiari syndrome (see later), and biliary obstruction, leading to abnormal attenuation and signal intensity adjacent to the portal triads.
Budd-Chiari Syndrome and Sinusoidal Obstruction Syndrome
The term Budd-Chiari syndrome is applied to a diverse group of conditions associated with hepatic venous outflow obstruction, at the level of either the large hepatic veins or the suprahepatic segment of the inferior vena cava. Diagnosis of this disorder is frequently difficult because of its protean causes and clinical manifestations. Sinusoidal obstruction syndrome is a subset of the Budd-Chiari syndrome in which nonthrombotic occlusion of the small presinusoidal venules occurs. This usually develops in the setting of chemotherapy, radiation therapy, and immunosuppressive therapy.
Clinical Findings
The two major clinical presentations of Budd-Chiari syndrome are determined by the extent and speed of onset of venous occlusion. The acute form follows simultaneous obstruction of all three hepatic veins or, more frequently, obstruction of the last patent hepatic vein after previous, clinically occult thrombosis of the others. These patients experience rapid onset of abdominal pain, tender hepatomegaly, vomiting, ascites, and arterial hypotension. This constellation of findings is associated with mild or marked increase of serum bilirubin level, marked elevation of the transaminase values, and decreased clotting factors resulting from hepatocellular failure.
Patients with the chronic form of Budd-Chiari syndrome present with ascites of insidious onset, right upper quadrant pain, hepatomegaly, normal or moderately increased transaminase values, decreased albumin and clotting factors, mild jaundice, and splenomegaly or variceal bleeding if cirrhosis develops. The diagnosis is seldom made on clinical grounds alone because clinicians tend to ignore the abdominal pain and ascribe the hepatomegaly and ascites to cirrhosis. The correct diagnosis may be inferred from the absence of a cause for the cirrhosis; episodes of abdominal pain during the preceding month; or, uncommonly, a palpable caudate lobe.
Etiology
Budd-Chiari syndrome is classified as either primary or secondary, depending on the cause and pathophysiologic manifestations ( Box 90-1 ). In the primary type, there is total or incomplete membranous obstruction of hepatic venous blood, either above the entrance of the hepatic veins into the inferior vena cava or between the ostium of the right hepatic vein, which remains patent, and the more superior middle and left hepatic veins. Membranous obstruction, although uncommon in Europe and North America, is the most common cause of Budd-Chiari syndrome in Japan, India, Israel, and South Africa. These membranes vary greatly in size, from wafer thin to several centimeters thick. The origin of this lesion is controversial; most are believed to be congenital malformations that result from deviations of the complex embryologic process by which the inferior vena cava is formed. Other theories suggest that some of these lesions are acquired secondary to mechanical injury, infection, phlebitis, or organization of a preexisting thrombus. This origin would explain the adult presentation of the disorder.
Hypercoagulable States
Antiphospholipid syndrome
Antithrombin III deficiency
Essential thrombocytosis
Factor V Leiden
Lupus anticoagulant
Myeloproliferative disorder
Paroxysmal nocturnal hemoglobinuria
Polycythemia rubra vera
Postpartum thrombocytopenic purpura
Protein C deficiency
Protein S deficiency
Sickle cell disease
Infection
Amebic liver abscess
Aspergillosis
Filariasis
Hepatic abscess
Hydatid cysts
Pelvic cellulitis
Schistosomiasis
Syphilis
Tuberculosis
Cancer
Adrenal cancer
Bronchogenic carcinoma
Fibrolamellar cancer
Hepatoma
Leiomyosarcoma
Leukemia
Renal cell cancer
Rhabdomyosarcoma
Miscellaneous
Behçet’s disease
Celiac disease
Crohn’s disease
Laparoscopic cholecystectomy
Membranous obstruction of vena cava
Oral contraceptives
Polycystic disease
Pregnancy
Sarcoidosis
Trauma
Membranous obstruction of the inferior vena cava is complicated by hepatocellular carcinoma in 20% to 40% of cases in South African and Japanese series. It is postulated that the obstruction renders the hepatocytes more susceptible to the action of one or more environmental carcinogens or that perhaps long-standing venous obstruction induces formation of hyperplastic nodules, which may undergo malignant transformation.
Secondary Budd-Chiari syndrome can be classified on the basis of the site of hepatic venous obstruction. Obstruction at the level of the central and sublobular veins may follow chemotherapy with thioguanine, vincristine, cytarabine, 6-mercaptopurine, or dacarbazine; internal or external hepatic radiation; chemoradiation therapy before bone marrow transplantation; azathioprine for immunosuppression; arsenic poisoning; pregnancy (particularly in the postpartum period); and oral contraceptive use. Naturally occurring toxins, such as the pyrrolizidine alkaloids found in herbal teas containing senecio and aflatoxins, have been implicated as well. Nonthrombotic occlusion of small central and sublobular veins is termed sinusoidal obstruction syndrome.
Sinusoidal obstruction syndrome (also known as hepatic veno-occlusive disease) is a distinctive and potentially fatal form of hepatic injury that occurs after drug and toxin exposure. Obstruction of the sinusoids occurs in central areas with hepatocyte necrosis and hemorrhage. Endothelial injury is the initiating event. Sinusoidal obstruction syndrome produces a hypercoagulable state. The venular and sinusoidal lumens are reduced because of edema and partial to complete fibrosis of venular lumens. Cyclophosphamide is the most common agent.
Obstruction of the major hepatic veins may occur in the setting of polycythemia vera, paroxysmal nocturnal hemoglobinuria, myeloproliferative disorders, thrombocytosis, hypereosinophilic syndrome, sickle cell anemia, Behçet’s disease, anticardiolipin antibodies, lupus anticoagulant, protein S or C deficiency, antithrombin III deficiency, antiphospholipid antibodies, mixed connective tissue disease, and Sjögren’s syndrome.
A variety of hepatic and extrahepatic masses can also compress the inferior vena cava and hepatic veins. These masses are the second most common mechanism of hepatic venous outflow block in Western countries. Hepatocellular carcinoma, hypernephroma, adrenal and bronchial carcinomas, leiomyosarcoma of the inferior vena cava, and hepatic adenoma or cystadenoma are the most common neoplasms associated with Budd-Chiari syndrome. Benign masses, such as hydatid cysts, intrahepatic hematoma, Caroli’s disease, large simple cysts, and amebic abscesses, can also cause obstruction.
Pathologic Findings
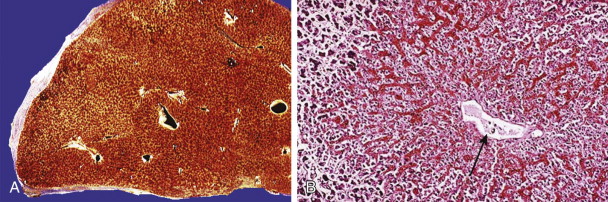
In acute Budd-Chiari syndrome, the liver is markedly enlarged and reddish ( Fig. 90-7A-C ). On histologic examination, sinusoidal dilation is evident, and parenchymal damage ranges from mild atrophy to marked hemorrhagic necrosis in the centrilobular areas.

In chronic Budd-Chiari syndrome, the liver is nodular and irregular in shape, often with caudate lobe hypertrophy. Centrilobular fibrosis is the hallmark of this disorder, and the centrally scarred areas may link to form connective tissue bridges. Sinusoidal dilation also predominates around hepatic venules and is associated with liver cell atrophy and perisinusoidal fibrosis.
In sinusoidal obstruction syndrome, the venular and sinusoidal lumens are reduced because of edema and partial to complete fibrosis of venular lumens ( Fig. 90-7D ).
Regenerative nodules are often seen in Budd-Chiari syndrome and are usually multiple, measuring between 5 and 40 mm. They develop in the setting of insufficient blood supply to the liver, leading to atrophy along with compensatory nodular hyperplasia in areas of hepatic parenchyma that have an adequate blood supply. The nodules are composed of hyperplastic hepatocytes arranged in plates of one or two cells in width. The nodules grow in an expansile fashion, compressing the surrounding liver and obliterating the central vein. There is no evidence that these nodules degenerate into malignancy.
Radiologic Findings
Traditionally, the radiographic “gold standard” for the diagnosis of Budd-Chiari syndrome has been hepatic venography and cavography. However, these invasive angiographic procedures are not suitable for screening of patients with nonspecific signs and symptoms. The cross-sectional imaging modalities have proved successful in the diagnosis of this disorder, and such studies can usually obviate angiography or venography.
Ultrasound
Because of its excellent sensitivity, high specificity, noninvasiveness, relatively low cost, availability, lack of contrast requirements, and multiplanar imaging ability, sonography can be the initial screening study for the Budd-Chiari syndrome. The sonographic features ( Fig. 90-8 ) of the Budd-Chiari syndrome are stenosis of hepatic veins (which often have thick, echogenic walls and proximal dilation), echogenic intravenous thrombi, intrahepatic collaterals or extrahepatic anastomoses, and large inferior right hepatic vein.

In chronic cases, the hepatic veins may not be visible. The inferior vena cava may also be narrowed by a grossly swollen liver. Ascites, abnormal hepatic shape with enlargement of the caudate lobe, and atrophy of the right lobe are present in most cases except in disease of acute onset. When both the left lobe and the caudate lobe are enlarged, confusion with cirrhosis can occur. Membranous webs are identified as echogenic or focal obliterations of the lumen.
In patients with hepatomegaly, evaluation of the hepatic veins can be difficult because they are often compressed and may not be visible. In these patients, an abdomen distended by ascites can further interfere with sonography. Similarly, in cirrhotic livers, patent hepatic veins may be difficult to identify, which precludes placement of the duplex Doppler cursor. Visualization of hepatic webs is also a problem. Color flow Doppler examination may be helpful in these cases.
Absent or reversed flow in the hepatic veins or flat flow in the hepatic veins associated with reversed flow in the inferior vena cava as demonstrated by duplex Doppler ultrasound is diagnostic of Budd-Chiari syndrome. The flow in the portal vein may be slow or reversed.
Color Doppler sonography overcomes some of the limitations of real-time sonography by demonstrating reversed flow in the retrohepatic inferior vena cava, documenting the presence of intrahepatic venous collaterals, and confirming the absence of flow in presumably thrombosed vessels. Color flow imaging rapidly and accurately determines the status of the hepatic veins and inferior vena cava and the direction of flow. Areas of occlusion are also clearly depicted, as are areas of narrowing, tortuosity, and reversal of flow direction.
In sinusoidal obstruction syndrome, there is nonthrombotic occlusion of small hepatic veins and terminal hepatic venules, the major hepatic veins show normal hepatofugal flow direction, and the inferior vena cava is patent with flow toward the heart. Decreased, reversed, or to-and-fro flow (flow demodulation) may be seen in the main portal vein. Other Doppler criteria include decrease in spectral density, reversed flow or maximum flow in the main portal vein of 210 cm/s, portal vein congestion index (cross-sectional area of vein divided by average velocity), hepatic artery resistive index of 0.75 or greater, monophasic flow in the hepatic veins, and flow recorded in paraumbilical veins. Gray-scale criteria include hepatic vein diameter of less than 3 mm, portal vein diameter of more than 8 mm in children and more than 12 mm in adults, and mural thickening of the gallbladder of more than 6 mm. Nonspecific findings include hepatosplenomegaly, ascites, and visualization of paraumbilical veins.
Computed Tomography
The CT features of Budd-Chiari syndrome depend on the age and extent of the obstruction and the presence of coexisting changes in portal venous blood flow. On noncontrast scans, in the acute phase of the disease, diffuse hepatic hypodensity is associated with global liver enlargement and ascites. The hepatic hypodensity is presumably due to hepatic parenchymal congestion. Hyperdense thrombus with an attenuation of 38 to 42 HU may be seen in the inferior vena cava and hepatic veins. Rarely, a calcified caval membrane is identified. After the injection of contrast material, the liver typically shows patchy enhancement; this is due to the hepatic congestion, which causes portal and sinusoidal stasis. In most patients, the central regions of the liver, including the caudate lobe and part of the left lobe, may enhance normally and appear hyperdense compared with the more peripheral parts of the liver, which show decreased enhancement. Later, a classic “flip-flop” pattern may develop ( Fig. 90-9 ), in which the contrast material from the normally enhanced central liver washes out so that this region becomes relatively hypodense compared with the peripheral zones, which are slowly accreting contrast material. The subcapsular portions of the liver may enhance normally because they have independent venous drainage through systemic subcapsular veins. These differences in hepatic attenuation and morphologic changes are closely related to regional disturbances in portal flow. In segments of the liver in which venous drainage is obstructed (the hepatic periphery), portal blood flow is diminished, if not reversed. Portal venous flow is normal in the central portion of the liver, where hepatic venous egress is uninterrupted.

Intravascular thrombus is best seen in the acute phase of the Budd-Chiari syndrome as a low-density mass in the lumen of the hepatic veins and inferior vena cava. The wall of the thrombosed vessel may appear dense relative to the thrombus secondary to the enhancement of the vasa vasorum. The involved vessel also is often enlarged. Portal vein thrombus may be seen in 20% of cases. Compression of the inferior vena cava is also demonstrated ( Fig. 90-10 ).

In more chronic cases of Budd-Chiari syndrome, the thrombi and the hepatic veins are often difficult to visualize. Intravascular thrombus density, which is high for the first 3 weeks, also diminishes over time. As the obstruction becomes more chronic, the liver undergoes morphologic changes that are due to diminished or reversed portal blood flow in the involved hepatic segments. Portal venous blood carries certain hepatotropic agents, mainly insulin, and segments deprived of these agents experience nutritional ischemia and eventually atrophy. Accordingly, the caudate lobe exhibits hypertrophy, and the periphery of the liver tends to exhibit atrophy. These changes may take 2 to 4 months to appear. Unless the membranes are calcified, membranous obstruction of the inferior vena cava is more difficult to visualize.
On MDCT, large regenerative nodules robustly and homogeneously enhance during the arterial dominant phase and remain slightly hyperdense on the portal venous dominant phase images as well.
Magnetic Resonance Imaging
MRI is a useful screening modality for Budd-Chiari syndrome by virtue of its ability to display directly the portal veins, hepatic veins, and inferior vena cava in numerous planes. A number of specific MRI abnormalities are associated with this syndrome ( Figs. 90-11 to 90-15 ): complete absence of hepatic veins or striking attenuation of their caliber, demonstration of thrombus or absent vascular flow in hepatic veins, comma-shaped intrahepatic collateral vessels, and marked constriction of the inferior vena cava. Less specific findings include enlargement of the caudate lobe, ascites, and inhomogeneity of the hepatic parenchyma. Vascular patency can confidently be confirmed when the vessel has no signal.





The multiplanar capabilities of MRI are of great value in depicting tumor thrombus in patients with Budd-Chiari syndrome secondary to neoplasm. Bland and tumor thrombus can be distinguished by the administration of gadolinium. Tumor thrombus may show contrast enhancement.
The degree of enhancement of the liver parenchyma supplied by a thrombosed hepatic vein depends on the acuteness and duration of the thrombosis. Involved parenchyma enhances less than surrounding liver in acute thrombosis. In chronic thrombosis, enhancement is more variable and may be increased. In patients with acute Budd-Chiari syndrome, the congested liver may have a higher water content and longer T2 relaxation time than the spared caudate lobe. Also, the peripheral liver enhances less than the central liver after intravenous administration of gadolinium because of increased parenchymal pressure with resultant diminished blood supply from both the hepatic artery and the portal vein.
Large regenerative nodules are bright on T1-weighted MR images and show the same enhancement pattern after the intravenous administration of gadopentetate dimeglumine (Gd-DTPA). They are predominantly isointense or hypointense relative to the liver on T2-weighted images.
Angiography
Angiography ( Fig. 90-16 ) has traditionally been the radiographic gold standard for the evaluation of patients with Budd-Chiari syndrome. Now it is seldom performed for diagnosis but as a preliminary procedure to radiologic interventions, such as transjugular intrahepatic portosystemic shunt (TIPS) creation, balloon angioplasty, or stricture stenting.

Nodular Regenerative Hyperplasia
On contrast-enhanced CT and MRI studies, multiple enhancing nodules are identified that are usually 5 to 7 mm in diameter ( Fig. 90-17 ).

Treatment
The treatment and prognosis of Budd-Chiari syndrome are determined by the rate and degree of hepatic outflow obstruction. In acute Budd-Chiari syndrome, survival time may be short because of acute liver failure or extension of thrombus into the inferior vena cava with resulting pulmonary emboli. Patients with chronic Budd-Chiari syndrome generally succumb to variceal bleeding secondary to severe cirrhosis and portal hypertension. Most patients require portal decompression to prevent liver failure and to resolve the ascites.
Treatment of Budd-Chiari syndrome depends on the cause. In some cases, medical management with large doses of steroids (for idiopathic granulomatous venulitis) or nutritional therapy (for an acutely enlarged, fatty liver compressing the inferior vena cava) may suffice. However, in most cases, medical therapy is limited and is directed toward anticoagulation and the control of ascites with diuretics.
Membranous obstructions of the inferior vena cava (see Fig. 90-16 ) and the hepatic veins are amenable to angioplasty with balloons or lasers and stent insertion. Before any intervention, these patients should receive anticoagulants because of the danger of pulmonary emboli from venous thrombi proximal to the membranes. This danger is the major risk of thrombolytic therapy as well. Angioplasty is more difficult in patients with extensive thrombus, but clinical improvement may accompany dilation and recanalization of only one hepatic vein.
Reports suggest the utility of expandable metal stents in the treatment of Budd-Chiari syndrome resulting from tumor compression or from idiopathic obstruction of the inferior vena cava. The TIPS procedure is useful in treating Budd-Chiari syndrome as well.
The surgical alternatives in Budd-Chiari syndrome are as follows: direct repair by membranotomy, membranectomy, or cavoplasty; portosystemic decompression by side-to-side portacaval or mesoatrial shunt; transplantation; and peritoneojugular shunt.
Liver in Cardiac Disease
Passive hepatic congestion is caused by stasis of blood within the liver parenchyma as a result of compromise of hepatic venous drainage. It is a common complication of congestive heart failure and constrictive pericarditis, wherein elevated central venous pressure is directly transmitted from the right atrium to the hepatic veins because of their close anatomic relationship. The liver becomes tensely swollen as the hepatic sinusoids engorge and dilate with blood ( Fig. 90-18 ). These changes may be transient, and full recovery follows once the patient’s congestive heart failure is corrected. In chronic right atrial failure, cardiac cirrhosis may ensue.
Clinical Findings
With acute congestion, the liver is enlarged and tender, and the patient may have severe right upper quadrant pain that is due to stretching of Glisson’s capsule. Patients typically have hepatojugular reflux on physical examination. Liver function abnormalities are often mild, but florid hepatic dysfunction simulating acute viral hepatitis may be seen. The diagnosis is usually not made clinically because the signs and symptoms of cardiac failure overshadow those of liver disease. In rare cases of cardiomyopathy, the patient may present with hepatic failure before cardiac disease is recognized as the cause of the hepatic dysfunction.
Radiologic Findings
Ultrasound
Ultrasound demonstrates hepatic enlargement and dilation of the inferior vena cava and hepatic veins in the early stages of this disorder ( Fig. 90-19 ). Normally, the maximal diameter of the main trunk of the right hepatic vein is 5.6 to 6.2 mm. In patients with congestive heart failure, the mean diameter is 8.8 mm, and it increases to 13.3 mm if there is associated pericardial effusion. Respiratory variations in the caliber of the inferior vena cava and hepatic veins are diminished.