Chapter Outline
Superior Mesenteric Artery Dissection
Drug-Induced Small Bowel Angioedema
Median Arcuate Ligament Syndrome
Small bowel abnormalities continue to be a significant diagnostic challenge for clinicians and radiologists. Clinicians have historically struggled to diagnose many small bowel diseases because patients typically present with only nonspecific complaints such as abdominal pain, weight loss, or anemia. Therefore, in most cases, the diagnosis of small bowel pathology is highly dependent on the radiologist. Although fluoroscopic barium studies have traditionally been the mainstay in the diagnosis of many small bowel diseases, they have proven to be inherently limited because they image only the lumen of the bowel and provide very little information regarding extraluminal disease. Since its introduction in the late 1970’s, computed tomography (CT) has proven to be extremely useful for the evaluation of the small intestine and has been used for the evaluation of such routine conditions as small bowel obstruction and Crohn’s disease. However, over the last decade, CT has taken on a larger role in diagnosing a variety of more subtle bowel diseases, including vascular disorders of the small intestine.
Vascular disorders of the small bowel encompass a wide variety of conditions, which primarily affect the mesenteric vasculature. These conditions have traditionally proven very difficult to diagnose radiographically, and ultimately angiography or surgery is relied on to make the correct diagnosis. With the introduction of multidetector CT (MDCT), along with the development of sophisticated three-dimensional imaging tools, it is now possible to image the small bowel vasculature, as well as the small intestine, in a single examination, offering a thorough evaluation in patients with a wide variety of suspected small bowel vascular disorders.
This chapter will review the current status of imaging regarding a variety of vascular disorders of the small intestine, including mesenteric ischemia and infarction, vasculitis, aneurysms, acute small bowel bleeding, and radiation enteritis. Although a variety of imaging modalities will be discussed, this chapter will concentrate on the use of MDCT and 3D imaging for the diagnosis of these conditions.
Computed Tomography Imaging
The last decade has brought significant improvements in CT technology with the development of 64-, 128-, and 256-slice and, most recently, dual-source scanners. These latest scanners have allowed markedly improved spatial and temporal resolutions, enabled the reliable acquisition of angiographic images at peak arterial enhancement, and reduced motion related artifacts. In addition to improving the image quality of conventional axial images and multiplanar reformats, these technologic advances have improved our ability to create truly isotropic data sets, and have greatly facilitated the creation of 3D reconstructions. These technologic improvements, along with the latest 3D software packages and CT protocol refinements, allow high-resolution images of the small bowel, small bowel mesentery, and mesenteric vasculature to be obtained, largely obviating the need for any other imaging modality.
When a vascular disorder of the small bowel is suspected, the CT examination must be specifically focused to optimize evaluation of the bowel. Prior to the injection of intravenous (IV) contrast, most patients are given a neutral oral contrast agent (e.g., barium sulfate [VoLumen, E-Z-EM, New York]) to distend the small bowel maximally, although in a few select cases (e.g., when acute gastrointestinal hemorrhage is suspected) no oral contrast agent is given. Notably, positive contrast agents are never given because a beam-hardening artifact from the contrast can obscure subtle bowel wall thickening or enhancement abnormalities and can also interfere with 3D postprocessing. An injection of roughly 120 mL of nonionic IV contrast is then administered at a relatively high rate of 3 to 5 mL/s to maximize arterial enhancement. Arterial phase images are acquired at 30 seconds after the injection of contrast, followed by the acquisition of venous phase images at 60 to 70 seconds. The arterial phase images are important in the evaluation of the arterial mesenteric vasculature, small bowel hypoenhancement or hyperemia, vascular malformations, and hypervascular small bowel tumors. The venous phase images are critical for evaluating the mesenteric venous vasculature, identifying subtle areas of bowel wall thickening or hypovascular small bowel tumors, and complete evaluation of the remainder of the abdomen and pelvis.
Images are acquired with thin collimation, with acquisition of 0.625- to 0.75-mm slices, which are then reconstructed into 3- to 5-mm axial slices for routine interpretation. Coronal and sagittal multiplanar reformations are created directly at most CT scanners immediately after acquisition of the axial source images. Simultaneously, a set of 0.5- to 0.75-mm isotropic” images are used to create 3D reconstructions.
Two primary 3D reconstruction methods, which are typically created interactively by a radiologist at an independent workstation, are the most useful for the evaluation of small bowel vascular disorders:
- 1.
Maximum intensity projection (MIP) imaging involves the acquisition of the highest attenuation voxels in a dataset and the projection of these voxels into a 3D display. These images are most useful for complete visualization of the mesenteric vasculature and for better visualization of abnormalities in the small bowel mesentery by accentuating soft tissue structures and vessels in the midst of mesenteric fat.
- 2.
Volume rendering (VR) is based on a complex algorithm that assigns a specific color and transparency to each voxel in a dataset based on its underlying attenuation and then presenting this data in a 3D display. This technique is the most useful for depicting abnormalities of the bowel wall itself and can accentuate subtle areas of bowel wall thickening or hyperemia.
- 1.
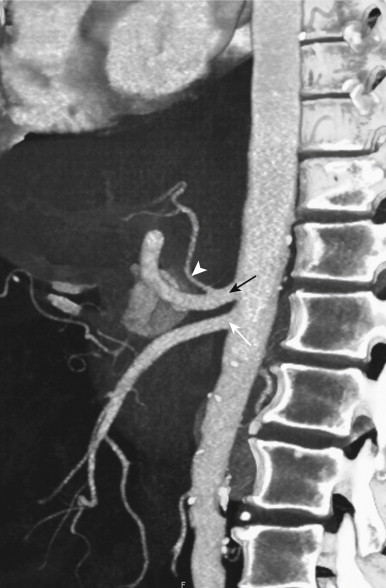
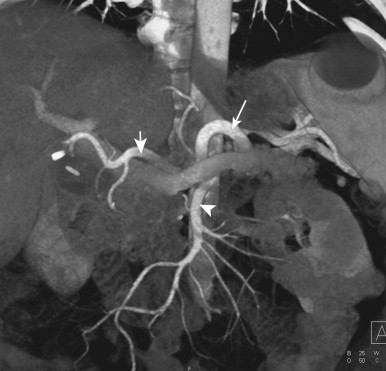
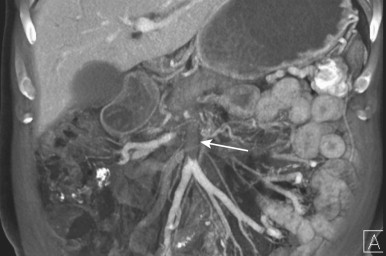
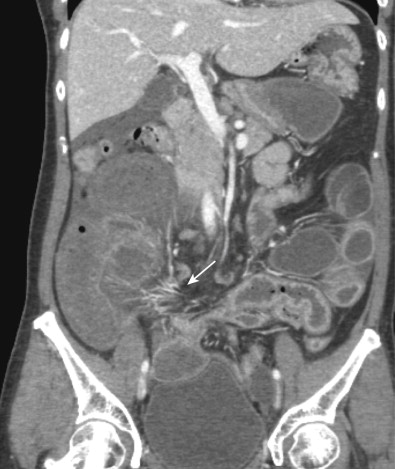
Because small bowel loops and the mesenteric vasculature are anatomically complex, and the relationships between different bowel loops and vascular structures are not easy to visualize using any single imaging plane, it is necessary to use a variety of imaging planes during 3D evaluation. To visualize the proximal portion of the celiac axis, superior mesenteric artery (SMA), and inferior mesenteric artery (IMA), the sagittal projection is often the most helpful ( Fig. 47-1 ). However, to visualize the complicated vascular branching of the arterial vessels adequately as they extend distally, coronal or coronal oblique planes are necessary ( Fig. 47-2 ). Similarly, the mesenteric veins are best seen in a coronal projection ( Fig. 47-3 ).
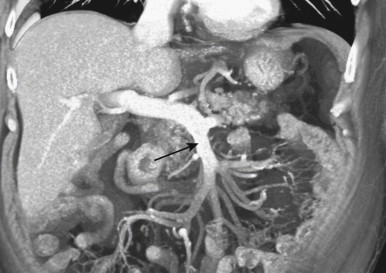
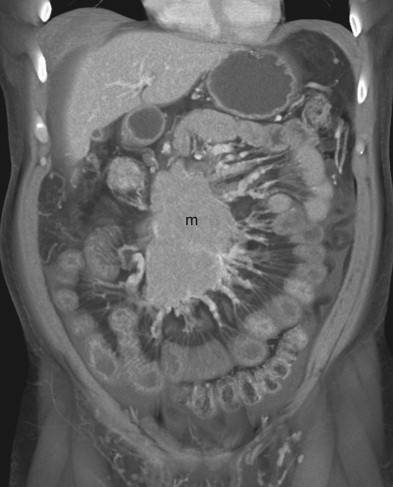
After adequate evaluation of the mesenteric vessels, it is also important to image the bowel ( Fig. 47-4 ) comprehensively. However, visualizing the small bowel in its entirety on the axial images can be difficult and time-consuming, and the full extent and distribution of bowel abnormalities can be difficult to appreciate. Moreover, in our experience, subtle abnormalities in the fold pattern of the small bowel are difficult to appreciate in the axial plane, and the terminal ileum and ileocecal valve tend not to be well visualized. Instead, the small bowel is best visualized in the coronal plane, using cut planes to remove overlapping bowel loops while performing 3D analysis.
Mesenteric Ischemia
Acute Mesenteric Ischemia
Accounting for roughly 5% of all hospital admissions, acute mesenteric ischemia (AMI) is a severe, life-threatening disorder, with mortality rates ranging from 60% to 80%. Unfortunately, AMI can be extremely difficult to diagnose clinically. Although patients complain of intense abdominal pain, their physical examination can be misleadingly unremarkable. Laboratory markers are rarely suggestive, although lactate levels can be elevated, and no single laboratory marker is absolutely specific. Ultimately, given the nonspecific presentation of most patients, the diagnosis is contingent on radiology.
A relatively large proportion of cardiac output is routed to the small bowel in the resting state and after a meal, making the small bowel extremely sensitive to decreases in blood flow. Acute mesenteric ischemia can be broadly divided into three major categories: (1) arterial occlusion; (2) venous occlusion; and (3) nonocclusive mesenteric ischemia.
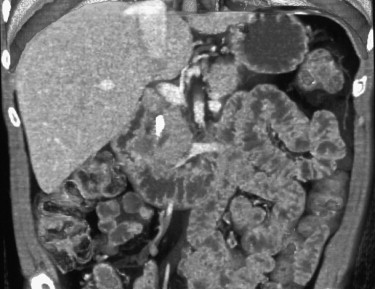
The most common cause is arterial occlusion, which accounts for 60% to 70% of all cases. Arterial occlusion can result from emboli to the SMA, usually in the setting of atrial fibrillation, but also secondary to recent myocardial infarction, mycotic aneurysms, and severe ulcerated plaque in the thoracic or abdominal aorta. The SMA is considered particularly vulnerable to emboli, given its wide caliber and narrow angle at takeoff, and a large embolus lodged at its origin can occlude blood flow to almost the entire small bowel and right colon. Most emboli lodge in the most proximal aspect of the SMA (usually 3 to 10 cm from the origin), although smaller emboli can travel distally and occlude blood flow to a smaller segment of the small bowel or right colon ( Figs. 47-5 to 47-8 ). Therefore, acute mesenteric ischemia should not be discounted when a relatively short segment of small bowel is involved, rather than the entire SMA territory.
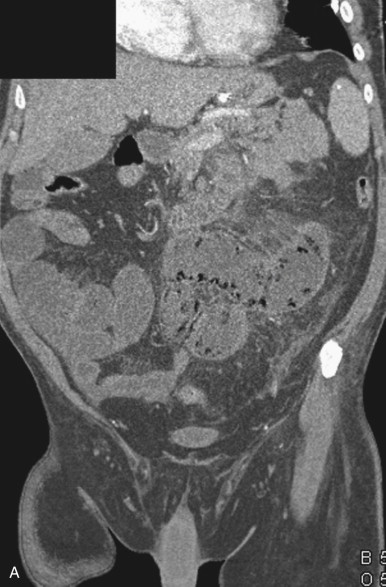



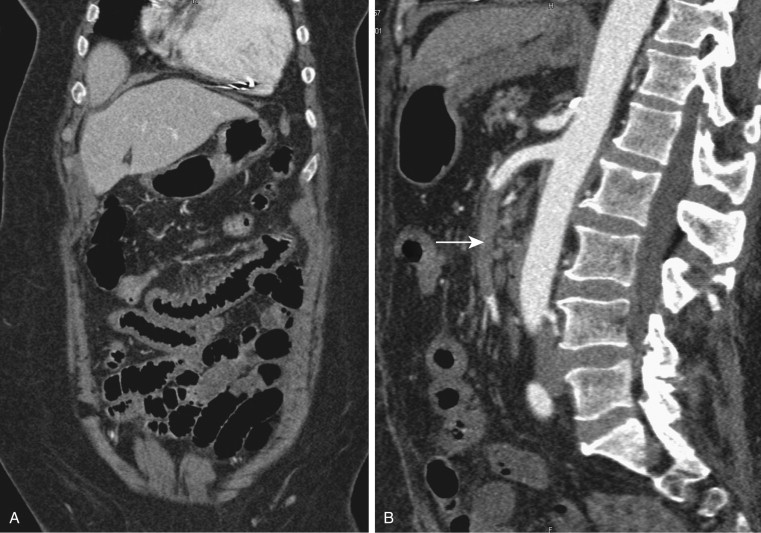
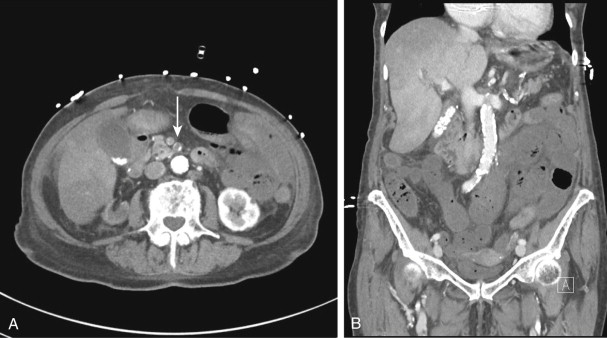
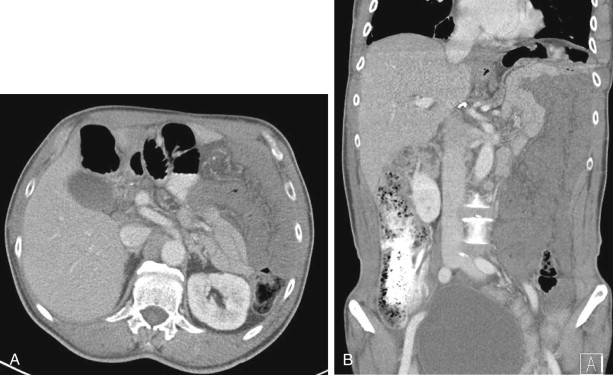
Although most arterial occlusive ischemia of the small bowel is embolic in nature, there are several other causes. Thrombosis of the SMA can occur as a result of severe atherosclerotic disease or in patients with underlying hypercoagulability syndromes. When thrombosis occurs in the setting of underlying atherosclerosis, the most common sites of occlusion are at the origins of the SMA and celiac artery. This particular subgroup of patients often has a history of chronic mesenteric ischemia, with development of AMI after superimposed thrombosis of a chronically narrowed and diseased vessel ( Fig. 47-9 ). Notably, although arterial collaterals are not usually found in patients with acute occlusive AMI, patients with a history of chronic mesenteric ischemia and superimposed arterial thrombosis can have evidence of arterial collaterals, a sometimes confusing feature. More rarely, acute mesenteric ischemia can be seen in the setting of vasculitis, aortic dissection (with extension of an occluding dissection flap into the SMA), occlusion of the SMA by surrounding tumor, or thrombosis of an SMA aneurysm ( Fig. 47-10 ).
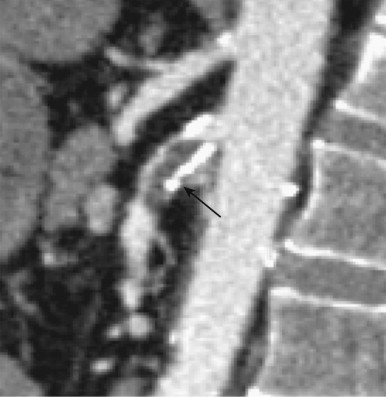
Venous occlusion (thrombosis of the superior mesenteric vein [SMV]) accounts for roughly 10% of all cases of AMI. A sizeable percentage of these patients are ultimately found to have a history of a specific hypercoagulability syndrome, personal or family history of unexplained thrombotic episodes, underlying malignancy, or oral contraceptive use, although a discrete cause for the venous occlusion is not found in at least one third of patients ( Figs. 47-11 to 47-13 ). Unfortunately, although the acute symptoms of venous occlusive AMI are usually much less severe compared to those of arterial occlusion, this can lead to considerable delay in presentation and diagnosis, resulting in a mortality rate as high as 40%.
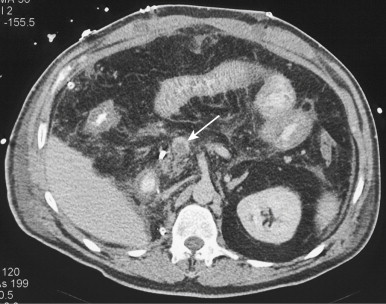

Complicated small bowel obstructions are also a cause of occlusive ischemia because strangulation of the bowel results in occlusion of the vasculature leading to the involved bowel segment. This type of bowel ischemia typically involves a combination of arterial and venous occlusion ( Fig. 47-14 ).
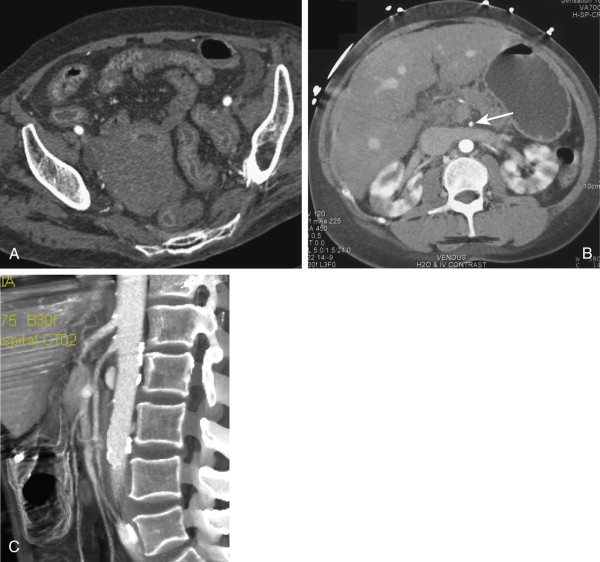
The final major category of acute mesenteric ischemia is nonocclusive ischemia, typically caused by hypotension and diminished blood flow to the small bowel resulting from underlying conditions such as cardiogenic shock, cardiac failure, acute myocardial infarction, severe hypovolemia, trauma, renal failure with overly aggressive dialysis, or severe vasoconstriction from drugs (e.g., digitalis, cocaine). Nonocclusive AMI accounts for roughly 30% of all cases of bowel ischemia and is associated with a 70% mortality rate. The odds of acute mesenteric ischemia in the setting of a low-flow state are increased when the patient has underlying atherosclerotic disease or some other abnormality of the mesenteric vasculature. In younger patients, nonocclusive mesenteric ischemia has been reported with cocaine use, which results in splanchnic vasoconstriction to preserve blood flow to the heart and brain ( Fig. 47-15 ).
Although the diagnosis of AMI was once dependent on angiography, CT has now assumed a primary role in the diagnosis and has proven to be highly effective. Several studies have shown CT to be extremely accurate, with sensitivities greater than 90%. A large meta-analysis studying the accuracy of CT in AMI found a pooled sensitivity of 93.3% and specificity of 95.9%. There is little doubt that CT should be considered the first-line radiologic examination when AMI is suspected.
Computed Tomography Findings
Bowel Dilation.
Small bowel dilation is common, although completely nonspecific. In a series of nine patients by Lee and colleagues, eight of nine patients demonstrated bowel dilation. In some cases, dilated bowel can be seen on the basis of an adynamic ileus and interruption of normal bowel peristalsis after an ischemic event and, in other cases, severe bowel dilation can be seen with irreversible transmural bowel infarction. In general, small bowel dilation is more common after venous occlusion than with arterial occlusion.
Bowel Wall Thickening.
Although not completely specific, bowel wall thickening is the most common CT finding in bowel ischemia, likely on the basis of edema and hemorrhage within the bowel wall. The normal small bowel wall is usually from 3 to 5 mm thick. In the study by Lee and associates, bowel wall thickening up to 1.5 cm was noted in patients with mesenteric vein thrombosis. However, it is important to remember that this finding is relatively nonspecific and can be seen in a great variety of other conditions.
Despite being a common finding in AMI, bowel wall thickening is usually seen after venous occlusion or thrombosis and is not a characteristic feature of AMI caused by arterial occlusion. In patients with AMI on the basis of arterial occlusion, the bowel wall is usually thinned, rather than thickened. Moreover, although small bowel wall thickening is a common finding in AMI, the presence and degree of bowel wall thickening do not usually correlate with the severity of ischemic damage.
Bowel Wall Attenuation and Abnormal Enhancement.
The appearance of the bowel wall can vary dramatically in cases of bowel ischemia, with a low-density wall usually reflecting submucosal edema; high density within the wall is usually secondary to intramural hemorrhage. Notably, intramural hemorrhage can be a very difficult finding to identify without the aid of noncontrast images because hemorrhage within the bowel wall can be confused with normal enhancement of the wall. Bowel wall edema and hemorrhage are most common after venous thrombosis, although they can also be seen in the setting of reperfusion after arterial occlusion or a nonocclusive AMI.
Small bowel ischemia can result in diffuse hypoenhancement of the bowel wall and mucosa, a halo or target appearance, or diffuse hyperenhancement. Diffuse hypoenhancement of the bowel wall is the most specific finding and is usually seen in the setting of acute arterial occlusion or diffuse transmural infarction, regardless of any cause. Diffuse hyperenhancement of the bowel wall or a halo appearance (with hyperenhancement of the bowel mucosa and diffuse, low-density edema of the submucosal layer) can also be seen with ischemia. This mucosal hyperenhancement is usually seen after venous occlusion but can also occur secondary to reperfusion after arterial occlusion or nonocclusive ischemia.
Rarely seen in some patients with ischemia, there can be delayed enhancement of affected loops, likely on the basis of delayed delivery of contrast, as well as contrast persisting in the ischemic segment because of delayed washout.
Stranding and Ascites.
Ascites and mesenteric stranding are nonspecific findings in patients with bowel ischemia. Their presence depends on the cause, duration, severity, and site of involvement of the bowel ischemia. In general, the presence of mesenteric inflammation and edema is not synonymous with bowel infarction and can be seen with venous occlusion and strangulated bowel obstructions without evidence of frank infarction. Moreover, hemorrhage in the mesentery is a very common finding in cases of venous thrombosis. However, in patients with arterial occlusion, infiltration and edema of the mesentery is a much more ominous finding, often seen in the setting of bowel infarction.
Pneumatosis and Portomesenteric Venous Air.
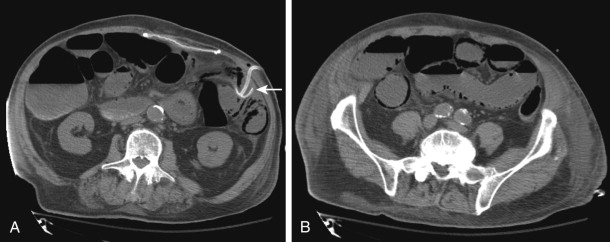
Pneumatosis and portomesenteric venous gas are relatively specific signs for transmural bowel infarction, although both are relatively rare ( Fig. 47-16 ). When the mucosal layer of the bowel is disrupted after an infarction, air can extend directly into the bowel wall, producing pneumatosis, seen in 6% to 30% of cases of acute mesenteric ischemia. This gas can then extend further into the mesenteric veins and portal veins (portomesenteric venous air), a finding that is only seen in 3% to 14% of cases.
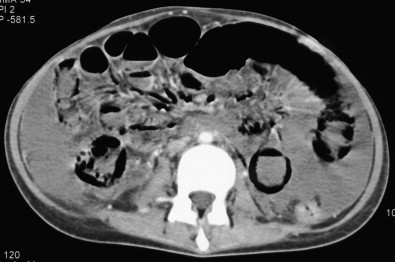
The specificity of pneumatosis and portomesenteric venous gas for ischemia and infarction approaches 100%. However, these two findings must be interpreted with a great deal of caution, because, rarely, they can be seen in a few nonischemic conditions as well, including iatrogenic mucosal injury (e.g., after the placement of a gastrostomy tube or jejunostomy tube), infections, bowel trauma, and inflammatory diseases ( Fig. 47-17 ). In addition, the radiologist must be careful not to confuse gas tracking along a bowel fold or gas immediately adjacent to the bowel wall with pneumatosis.
Patterns of Ischemia: Arterial Occlusive Ischemia Versus Venous Occlusive Ischemia.
Although there can often be a great deal of overlap, the pattern of abnormalities seen on CT secondary to arterial occlusion and venous occlusion can vary. Although bowel wall thickening is commonly thought to represent a critical imaging finding in bowel ischemia, this finding is less commonly seen in cases of arterial occlusion. Rather, the bowel actually tends to become thinned (paper thin bowel) because there is a lack of arterial flow but no appreciable mural edema or intramural hemorrhage. This thinning can also be attributed to loss of intestinal muscular tone after the ischemic event. In most cases of arterial occlusion, hypoenhancement of the small bowel wall and mucosa is present, although hyperemia of the mucosa can rarely be seen in cases of reperfusion after an embolic event. Acutely, the bowel is not usually dilated unless there is evidence of infarction. Finally, there is generally a relative paucity of mesenteric fluid, hemorrhage, and fat stranding, although these three features can be seen when there has been progression to frank bowel infarction. Given these findings, the CT appearance of acute AMI in the setting of arterial occlusion can be relatively difficult to identify; careful attention must be paid to subtle changes in bowel enhancement when an embolus is identified in the SMA.
The CT appearance after venous thrombosis is generally much more impressive, as the bowel wall is usually markedly thickened, the wall can be diffusely hypointense because of edema or hyperintense because of intramural hemorrhage, and the mucosa is often avidly hyperemic. The bowel can sometimes be moderately dilated, and there is typically significant mesenteric hemorrhage, edema, fluid, and fat-stranding, even in the absence of true bowel infarction.
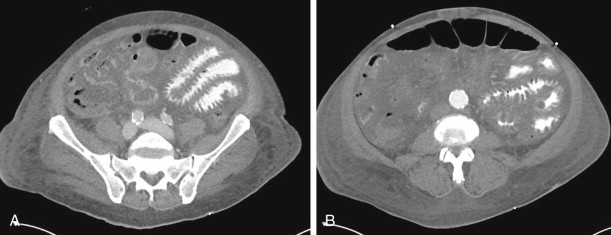
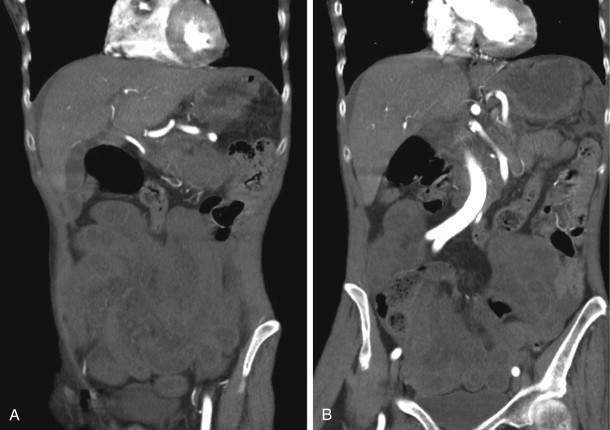
As one would expect, complicated bowel obstructions demonstrate elements of venous and arterial occlusion, depending on the degree of strangulation, and can have a variable appearance on CT, depending on which vessels are compromised. Nonocclusive mesenteric ischemia does not have a characteristic pattern and can be the most difficult of the three types of AMI to diagnose ( Figs. 47-18 and 47-19 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree