CHAPTER 112 Vascular Imaging of Renal and Pancreatic Transplantation
The use of kidney transplantation to treat end-stage renal disease in the United States has steadily increased from 43/million in 1996 to 55.5/million in 2005. The most recent data (2006) show that there are now more than 103,000 Americans living with a renal transplant and more than 9,400 with a pancreas or kidney-pancreas transplant, up from 55,000 and 4,000, respectively, in 1996.1 Imaging plays a major role in the surveillance of transplant complications. In this chapter, the vascular complications associated with renal and pancreatic transplantation are discussed.
VASCULAR EVALUATION OF RENAL TRANSPLANT RECIPIENTS
Transplant Renal Artery Stenosis
Prevalence and Epidemiology
TRAS has been reported in 1% to 23% of cases, with most series finding 2% to 10% of renal transplants are complicated by TRAS.2,3 It is usually a relatively late complication, occurring from 3 months to 2 years or more after transplantation.
Etiology and Pathophysiology
TRAS is most common at the anastomosis or most proximal segment of the donor artery and the risk is directly related to technique. Cadaveric transplants are typically harvested with an aortic patch from which the single or multiple renal arteries arise. The patch can then be anastomosed with its arterial origins end to side on the external iliac artery. Grafts from living donors, who cannot sacrifice an aortic patch, are typically anastomosed end to end to the internal iliac artery. When living donor grafts with multiple renal arteries must be used, the accessory arteries may be reconstructed to flow from the main renal artery, anastomosed separately, or anastomosed to the inferior epigastric artery (Fig. 112-1). End-to-end anastomoses have a threefold greater risk of stenosis.
Contributing causes of stenosis in end to end anastomoses are thought to be abnormal fluid dynamics and abrupt changes in caliber. Other more general causes or precipitants of stenosis include faulty suture technique, clamp injury, and kinking of the artery. In addition, atherosclerosis can occur or progress in the donor artery. An association with stenosis has also been shown among patients who have experienced episodes of acute rejection possibly caused by a component of endothelial injury from rejection of the graft artery. An additional association with cytomegalovirus infection has been reported.4 Finally, a stenosis of the recipient artery, usually the external iliac or common iliac artery, proximal to the anastomosis may have hemodynamic effects on the kidney, similar to stenosis of the graft artery.
Manifestations of Disease
Imaging Techniques and Findings
Ultrasound
Like native renal artery stenosis, the diagnosis of TRAS is made with ultrasound by demonstrating a focal area of aliasing on color Doppler imaging from turbulence caused by the stenosis and an elevated peak systolic velocity through the stenosis more than 2.5 m/sec on spectral Doppler imaging (Fig. 112-2).5 Because of the proximity of the allograft to the body wall in the anterior pelvis, the main artery can often be visualized in its entirety, aiding the chances of confirming the diagnosis. Power Doppler, with its high sensitivity to flow in all directions, is helpful to localize and map the artery for further evaluation with color Doppler and placement of the spectral Doppler gate. However, transplant arteries are typically much more tortuous than native renal arteries. This makes the setting of accurate angle correction on spectral Doppler more difficult and sometimes almost impossible. Furthermore, a range of elevated velocities has been shown in transplant arteries, even when stenosis is not suspected or proven not to be present, particularly if there is tortuosity.6 When there is no significant curvature, an elevated velocity may reflect elevated velocity in the external iliac artery. In these cases, a ratio of velocities in the main transplant artery and external iliac artery less than 1.8 is unlikely to be stenosis.
As in native renal stenosis, the characteristic parvus-tardus waveform of the intrarenal arteries downstream from a stenosis can support the diagnosis. This is reflected in spectral broadening of the arterial waveform, retarded acceleration less than 1.5 m/sec2, and increased acceleration time more than 0.08 to 0.10 second (Fig. 112-3) However, these findings have been shown to be less specific than peak systolic velocity and should be used to support the diagnosis based on peak systolic velocity.7 Resistive and pulsatility indices can also be lowered in TRAS but are only nonspecific indicators of graft dysfunction.
Magnetic Resonance Imaging
MRI can demonstrate TRAS with a high degree of accuracy. In multiple series, gadolinium (Gd)-enhanced three-dimensional MRA has been shown to have a sensitivity of 67% to100% and a specificity of 75% to 100% for hemodynamically significant stenosis (Table 112-1). Although technically challenging on older scanners, the examinations have become routine with state of the art 1.5-T scanners.
Review of source images, as well as review of three-dimensional postprocessed projectional views, is necessary to make the appropriate diagnosis. MIPs and multiplanar reformations using variable thicknesses allow for direct visualization through the lumen of the transplant renal artery, the anastomosis, the iliac arteries, and usually the first-order branch arteries (Fig. 112-4). It is the ability to manipulate the volumetric data of MRA that makes it so effective. This is especially true in tortuous vessels, which are typical in renal transplants. Shaded surface volume renderings can be especially helpful to visualize the anatomy in the case of tortuous vessels.
An effective complement to Gd-enhanced MRA is three-dimensional phase contrast MRA. This sequence achieves the MR signal, and therefore contrast, from protons moving across flow-encoding gradients. The signal is velocity-encoded at a certain upper threshold so that superfast flow at severe stenoses nulls the signal. Although the sequence acquisition time is long (typically 5 to 8 minutes), phase contrast MRA can be performed on a small field of view, either in the area of the transplant renal artery or any area of suspicion during imaging. Confirming suspected stenoses by demonstrating loss of signal on phase contrast helps eliminate false-positives. The combination of Gd-enhanced MRA and phase contrast MRA has been shown to increase specificity from 88% to 100%.8
The limit of MRA resolution for stenosis is typically at the level of the segmental first-order branch vessels. DSA is superior in its ability to detect segmental artery stenosis. Furthermore, intrarenal arteries are typically obscured by enhancement of the renal parenchyma with MRA. However, because MRA is an anatomic image that directly displays the vessels, similar to digital subtraction angiography (DSA), it offers many advantages over the detection of secondary findings of TRAS with Doppler ultrasound (US). For example, kinked or tortuous arteries that are technically challenging or impossible with Doppler US can be evaluated with the volumetric data sets of MRA. Iliac artery stenosis proximal to the transplant artery anastomosis is an uncommon cause of TRAS (pseudo-TRAS) that is much more easily identified with MRA (Fig. 112-5). Because of the shortage of grafts, en bloc transplantation of two kidneys of limited function such as from the cadavers of older patients or two small kidneys from pediatric cadavers, has gained acceptance (see Fig. 112-1C). In these cases, not only is the anatomy more complicated, but secondary findings of velocity or ratios of velocities have not been proven to be reliable indicators of TRAS. Direct anatomic imaging also allows for the detection of other pathologies that may be the cause of patient symptoms, such as infarctions, artery or vein thrombosis, urinary collecting system complications, or a perinephric collection. It can also be helpful for preoperative planning, even when DSA is already indicated.
Nuclear Medicine and Positron Emission Tomography
Although a decline in renal function after the administration of an ACE inhibitor can indicate TRAS, an ACE inhibitor challenge during scintigraphy is relatively contraindicated because of reports of ACE inhibitor–induced acute renal failure, in some cases irreversible. Additionally, the specificity is less than in US, MRA, and DSA.9

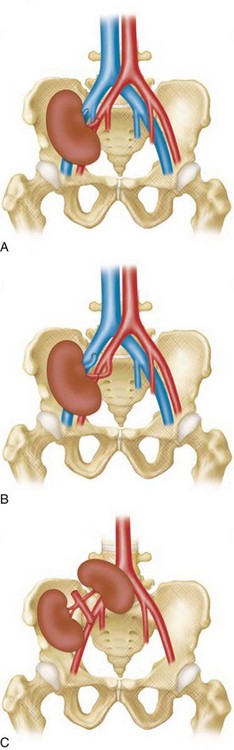
 FIGURE 112-1
FIGURE 112-1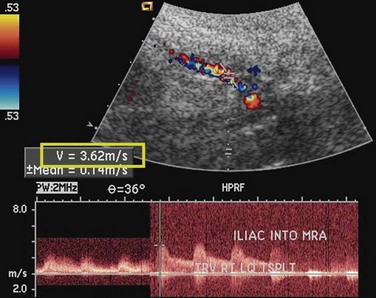
 FIGURE 112-2
FIGURE 112-2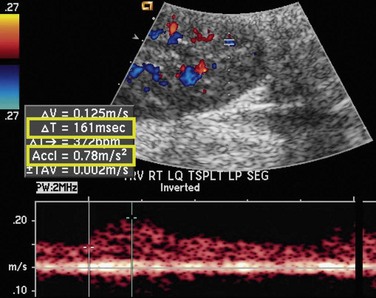
 FIGURE 112-3
FIGURE 112-3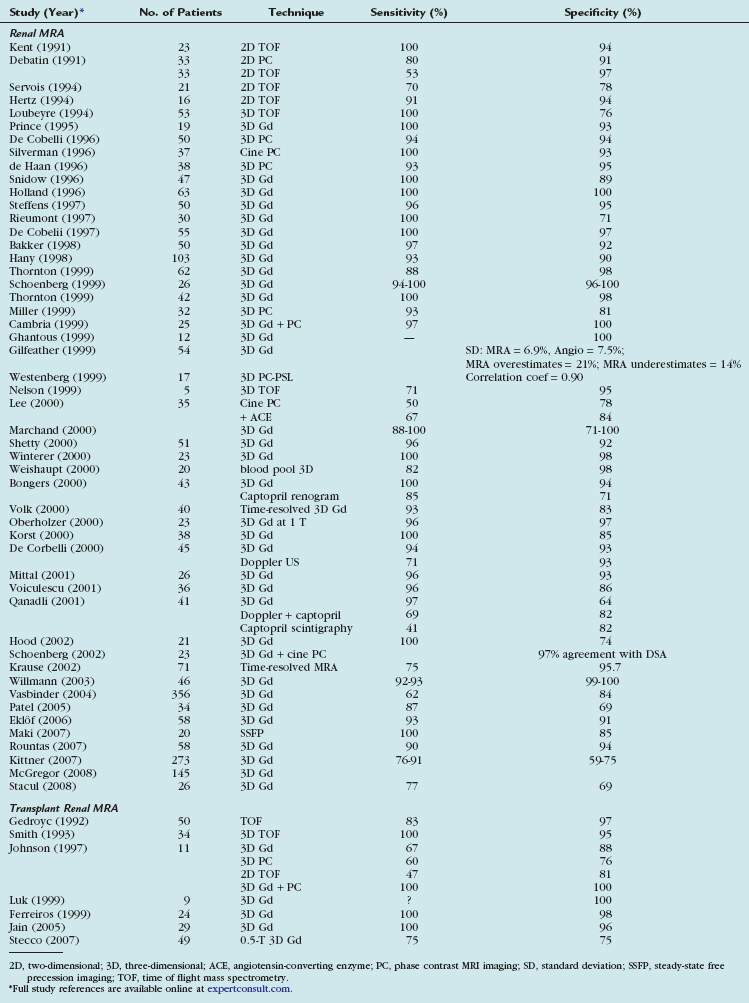
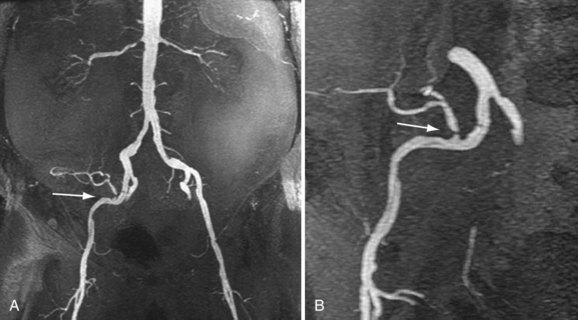
 FIGURE 112-4
FIGURE 112-4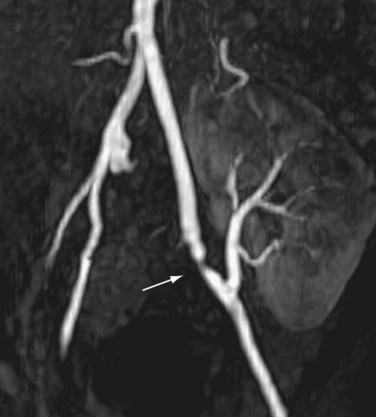
 FIGURE 112-5
FIGURE 112-5

