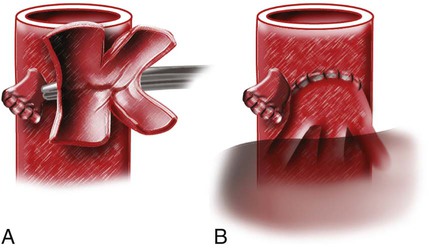
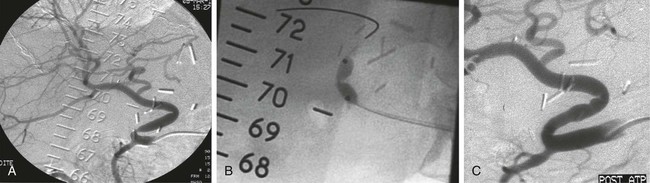
Julien Auriol, Philippe Otal, Marie Agnès Marachet, Thomas Lemettre, Bogdan Vierasu, Francis Joffre and Hervé Rousseau Orthotopic liver transplantation (OLT) consists of removing the damaged liver and replacing it with a graft in the recipient’s native bed.1 Heterotopic liver transplantation is when the graft is placed in an extrahepatic site, usually at the root of the mesentery, but is no longer used, owing to poor outcomes. Auxiliary liver transplantation is placement of the donor liver in the presence of the native liver. Such transplants may be either orthotopic (after removal of part of the native liver and placement of a portion of the donor liver) or heterotopic. Since 1989, another technique known as the piggyback technique is more and more used. The major hepatic veins of the recipient are clamped and interconnected, forming a cuff that can then be anastomosed to the suprahepatic vena cava of the donor liver in an end-to-end or end-to-side fashion (Fig. 70-1). This technique preserves the recipient retrohepatic vena cava and avoids vena caval clamping, preserving venous return during the operation and avoiding venovenous bypass. Arterial anastomosis between the donor and recipient arteries is usually end to end. The site usually varies depending on the arterial anatomy of donor and recipient and on the surgeon’s preference. One must recognize that patients with anomalous hepatic arterial anatomy may not have a large enough common hepatic artery to use as inflow. Patients with celiac axis stenosis may also have inadequate inflow. The median arcuate ligament syndrome has been described as affecting arterial inflow in liver transplantation. In these circumstances, use of a donor iliac arterial conduit from the infrarenal (or occasionally supraceliac) aorta to the allograft may be necessary. However, artificial conduits should be avoided, owing to the risk of thrombosis and infection. Biliary connections involve either a primary duct-to-duct technique (choledochocholedochostomy, the most common used) or require a choledochojejunostomy (to a Roux-en-Y defunctionalized intestinal loop). Duct-to-duct anastomosis is usually done over a T-tube. Advantages of leaving a T-tube are (1) observation of bile production and its quality as a sign of hepatic allograft function and (2) easy cholangiographic access to the biliary system in cases of abnormalities in liver function tests. The disadvantage of the T-tube is the risk of bile leak after removal of the tube, requiring emergency endoscopic retrograde cholangiopancreatography and decompression of the duct. Other types of biliary reconstructions, including choledochoduodenostomy and cholecystoduodenostomy, have been used through the years. Biliary complications of liver transplants are discussed more fully in Chapter 139. Hepatic artery stenosis occurs in 5% to 13% of transplants (one third in the first month), with a mean delay between diagnosis and transplantation of 100 to 126 days. The mechanisms and predisposing factors for this complication are failure of the surgical technique related to a small (children) or diseased artery, excessive vessel length with kinking and angulations, retransplantation, anatomic variation of the hepatic artery, and arterial reconstructions.2–4 Excluding conduits, the majority of hepatic artery stenoses are anastomotic (46%-75%), with distal stenoses representing 40% to 46%. In 3% to 8% of cases, stenoses are proximal to the anastomosis in the recipient artery. Multiple stenoses are found in 5% to 25%, and in the majority of cases (77%) anastomotic stenosis coexists with distal stenosis.2–5 Clinical presentation is variable and goes from asymptomatic with minimal increase in liver function test results (20% of cases) to acute liver failure or biliary complications. Laboratory findings are nonspecific and insidious.2 Doppler ultrasonography can allow early diagnosis. Values considered indicative of hepatic artery stenosis are a resistive index less than 0.5 and a systolic ascending time greater than 100 ms, or both. The right and left hepatic arteries must be evaluated to detect distal stenosis. A protocol for management of hepatic artery stenosis has been proposed by Saad et al.,5 who recommend a combination of surgery and percutaneous transluminal angioplasty (PTA). Hepatic artery PTA is reserved for solitary focal stenosis and nonsurgical candidates with other types of lesions (tandem lesions, arterial kinking). Repetitive endoluminal therapy is also required for post-PTA restenosis. Surgical revision is proposed for technical failure of PTA and hepatic artery thrombosis. Angiography is performed with standard catheter technique, mostly from a transfemoral approach, with 5F catheter (Simmons or cobra). A long sheath up to the origin of the feeding vessel may be useful. The size of the balloon ranges from 4 to 6 mm and can be chosen according to the automatic measurement, using the sheath as a reference. Heparin is administered after crossing the lesion with a 0.014- to 0.018-inch guidewire. Some authors advocate primary stenting to reduce the risk of acute thrombosis or dissection.3 Recent technical improvements make the procedure safer, such as monorail material or use of coronary stents (Fig. 70-2). Traditionally, the treatment of symptomatic hepatic artery stenosis was surgical and included revascularization and even retransplantation. In the past 20 years, numerous authors have described endoluminal treatment.2,3 Nevertheless, treatment of hepatic artery stenosis is debated because large numbers of patients are asymptomatic. In fact, if not treated, hepatic artery stenosis doubled the rate of biliary complications, thus reducing graft life expectancy. Furthermore, there is a progression from stenosis to thrombosis: the hepatic artery thrombosis rate for untreated significant hepatic artery stenosis is 65% at 6 months,5 and hepatic artery thrombosis is associated with higher morbidity and mortality than hepatic artery stenosis. Patency of the hepatic artery is crucial because vascularization of the biliary tree of the transplanted liver relies only on it, as opposed to the native liver in which a rich network of collateral arteries may compensate for hepatic artery occlusion. Improvements in symptoms and results of liver function tests have been demonstrated after treatment of the stenosis.2–4,6–9 Graft survival and complication rates in those patients treated by radiologic or surgical interventions matched those with normal arterial inflow.2 As mentioned earlier, kinking of the hepatic artery contraindicates hepatic artery PTA. Hepatic artery angioplasty has a technical success rate up to 81%.4,5 Causes of failure are hepatic artery tortuosity and inability to cross the lesion. Restenosis rates after hepatic artery PTA and stent placement are the same (≈30%), but restenosis of stents usually occurs later than restenoses in patients treated with balloon dilatation alone. Primary restenosis occurred at a mean interval of 2.7 months from initial PTA and 5.3 months after stent placement.5 All the data about follow-up patency rates are based on Doppler ultrasonography, which is not the gold-standard test for evaluation of hepatic artery stenosis. The rate of immediate complications (rupture and dissection) ranges between 7% and 9.5%.4,5 Hepatic artery thrombosis rates at 30 days and 1 year are 5% and 19%, respectively.5 Postprocedural anticoagulation and antiplatelet therapy may be beneficial in reducing this rate, but further investigations are required.5 Hepatic artery thrombosis is a rare but serious complication after liver transplantation, requiring retransplantation in almost 50% of patients.10 It occurs in 2.5% to 6.8% after OLT,10–12 and this incidence increases 5.8-fold when the donor hepatic artery was reconstructed with an interposition graft to the supraceliac aorta.10 Risk is increased in children (11%-26%) but is claimed to have less consequence, mainly if it occurs late after transplantation.13–17 Celiac trunk compression by the median arcuate ligament and hepatic artery stenosis are also described as predisposing factors for hepatic artery thrombosis. Hepatic artery thrombosis is classified as early or late by its occurrence within or beyond 30 days after OLT. Early hepatic artery thrombosis represents 33% to 46%10,11 of patients and may have a mortality rate as high as 55%, whereas it decreases to 15% if thrombosis occurs after this period.11
Vascular Intervention in the Liver Transplant Patient
Technical Features
Arterial Complications
Hepatic Artery Stenosis
Indications
Technique
Controversies
Outcomes
Complications
Hepatic Artery Thrombosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Vascular Intervention in the Liver Transplant Patient