23
Vascular Manifestations of Renal Disease
This chapter deals with diseases of the kidneys for which angiography or percutaneous transcatheter vascular interventional procedures play a major role. Nonneoplastic entities are discussed first.
 Renovascular Hypertension
Renovascular Hypertension
The interventional radiologist often is called on to help diagnose or treat renovascular hypertension. Renovascular hypertension is hypertension that is caused by arterial inflow reduction, which in turn causes renal ischemia and a secondary increased renin production. The mechanism of renovascular hypertension is believed to be as follows. When blood flowto the kidneys is limited to the point that it causes renal ischemia, glomerular filtration pressure drops, resulting in decreased filtration of blood by the kidney. Cells in the juxtaglomerular apparatus are stimulated to secrete renin, which causes elevation of the blood pressure, increasing the perfusion to the ischemic kidney, correcting the ischemia, and increasing the glomerular filtration pressure. The physiologic mechanism of renovascular hypertension is summarized in Figure 23-1.1
It must be kept in mind that hypertension related to excess renin secretion can have other causes. For example, it can be the result of chronic renal inflammation, which results in renal parenchymal insufficiency, or, rarely, a renin secreting tumor (a reninoma or juxtaglomerular tumor).
Etiology of renal artery occlusive disease
Renal artery occlusive disease can result from several disease processes. The two most common causes of renovascular hypertension are atherosclerosis and fibromuscular dysplasia (FMD).
Atherosclerosis is the most common cause of renovascular hypertension, accounting for approximately 60% of such cases.2 Most patients are older men, usually aged over 50 years. A relatively large percentage (37%) of patients with atherosclerotic renovascular hypertension will have bilateral renal artery stenosis.3 Most atherosclerotic renal artery stenoses are located in the proximal third of the renal artery and frequently involve the renal artery origin secondary to overhanging aortic mural atherosclerotic plaques (Fig. 23-2). This pattern of disease is relevant to the efficacy of balloon angioplasty in treating atherosclerotic renal artery stenosis.
FMD is the cause of renal artery stenosis in approximately one third of patients with renovascular hypertension.2 Unlike atherosclerotic stenoses, stenoses of FMD tend to occur in children and young adults, and the lesion tends to affect the mid to distal main renal artery and in some cases its branches. Branch vessel disease is quite common in FMD-induced renovascular hypertension in children. There are several types of FMD based on their pathology (Table 23-1).2 The most common variety encountered in practice is medial fibroplasia, which is caused by areas of circumferential medial thickening alternating with areas of aneurysm formation. Angiographically, this type manifests as a “string of beads” appearance (Fig. 23-3).
Other less common causes of renovascular hypertension include chronic aortic dissection with impingement on the renal artery ostium by the intimal flap, aortoarteritis such as Takayasu’s disease, neurofibromatosis, renal artery aneurysm, atheromatous and cholesterol emboli, postoperative stenosis at a previous bypass graft anastomosis or renal transplant artery anastomotic stenosis, and coarctation of the aorta with renal artery stenosis.4
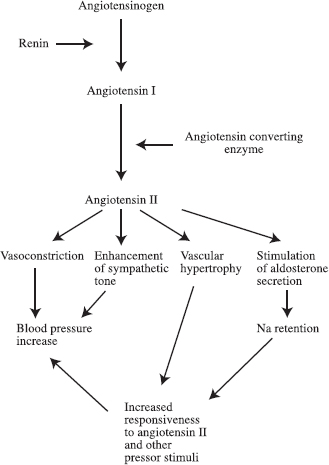
FIGURE 23-1. Physiologic mechanism of hypertension.
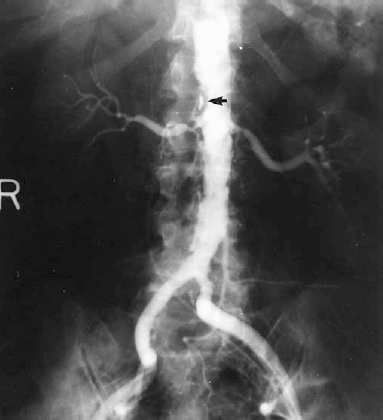
FIGURE 23-2. Bilateral atherosclerotic proximal renal artery stenoses: moderate on left, severe on right. Note the diffusely diseased abdominal aorta and prominent plaque immediately superior to right renal artery ostium (arrow) .
| Type | Prevalence | Angiographic findings |
| Medial fibroplasia | 60–70 | “String of beads” caused by focal stenosis alternating with aneurysmal dilatation |
| Perimedial fibroplasia | 15–25 | Chain of irregular stenoses without aneurysms |
| Medial hyperplasia | 5–15 | Long, smooth narrowing Medial dissection 5 False channel, aneurysm |
| Intimal fibroplasia | 1–2 | Short, focal stenosis |
| Adventitial fibroplasia | <1 | Long, segmental stenosis |
| From Kadir S. Diagnostic angiography. Philadelphia: WB Saunders, 1986:459. | ||
Diagnosis
Of the entire hypertensive population, fewer than 5% will have renovascular hypertension. The remaining 95% have essential hypertension, which is not attributable to any specific anatomic abnormality and thus is treatable only by long-term medication. Long-term medication for hypertension often involves multiple medications that have possible side effects. For this reason, detection of a correctable anatomic abnormality is a desirable goal.
Initially there was a great deal of enthusiasm for screening all hypertensive patients for renovascular hypertension, but, given the low prevalence of renovascular hypertension, even with a test of moderately good sensitivity and specificity, the frequency of false-positives equals or exceeds the frequency of true-positives.5 Invasive examinations, although capable of diagnosing renal artery stenosis as the cause of a patient’s hypertension with a higher degree of certainty, are not appropriate as screening examinations because of their cost and morbidity. These problems led to the abandonment of indiscriminate screening for renovascular hypertension.
The current approach to screening patients for renovascular hypertension involves screening only those hypertensive patients who, based on clinical parameters, are most likely to have renoocclusive disease as an etiology. These parameters include abrupt onset of hypertension before age 30 or after age 55, severe hypertension (diastolic blood pressure greater than 120 mm Hg), accelerated or malignant hypertension with retinopathy, hypertension refractory to triple-drug therapy, epigastric bruit, moderate hypertension with unexplained azotemia, and azotemia induced by angiotensin-converting enzyme (ACE) inhibitors.6
Radionuclide renography, Doppler ultrasound velocity measurements, and Doppler renal-resistive index measurements are among the noninvasive modalities used in the diagnosis of renovascular hypertension. Each modality has its limitations, but each also has some value in screening carefully selected persons.7–9
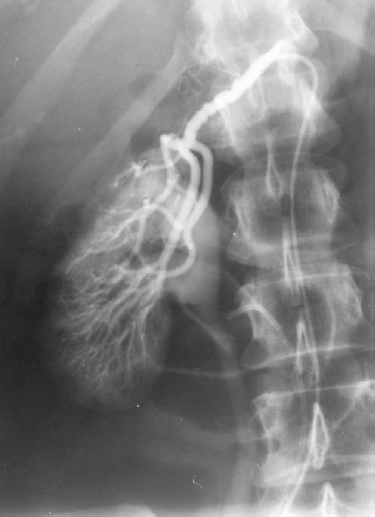
FIGURE 23-3. Fibromuscular dysplasia, medial fibroplasia type, of right renal artery. Note the “string of beads” appearance of the main artery.
Magnetic resonance angiography
Magnetic resonance angiography (MRA) shows great promise as a noninvasive modality for visually depicting the renal arteries and aorta. The available types of image acquisition techniques include two-dimensional (2-D) time-of-flight (TOF), 2-D or 3-D phase-contrast, and 3-D TOF, with or without intravenous gadolinium. The goal of MRA acquisition is to suppress signal from surrounding nonvascular structures and either to enhance or at least limit the suppression of flowing blood signal, which, in effect, produces a “subtraction arteriogram.” The resultant image data set can be reconstructed in a 3-D image depiction that can berotated and viewed from multiple angles.
MRA is reasonably sensitive and specific in depicting the aorta and proximal renal arteries. It is less sensitive for defining the distal main renal artery or beyond the division points of the main renal artery.10,11 Renal MRA has proved extremely valuable in the evaluation of the patient in whom there is a reasonable clinical suspicion of renovascular hypertension but in whom renal insufficiency poses a relative contraindication to contrast arteriography. A negative MRA virtually excludes a central renal artery lesion as the cause of the hypertension or the cause of acute onset of renal insufficiency (Fig. 23-4).
Spiral CT angiography
Spiral computed tomagraphy angiography (CTA) also holds promise as a modality for evaluating the renal arteries because of its relative noninvasiveness compared with catheter arteriography. The premise behind CTA is the rapid acquisition of axial images in the areas of interest in a single breath-hold, made possible by slip ring technology. The axial images can be reconstructed into a 3-D model of the arterial system that can be viewed from different angles.12 The disadvantage of CTA is the need for intravenous iodinated contrast, which limits its usefulness in certain patient populations, especially in patients with renal insufficiency or renal transplant artery stenosis. Often the amount of contrast needed for CTA far exceeds that required for a properly performed catheter arteriogram.
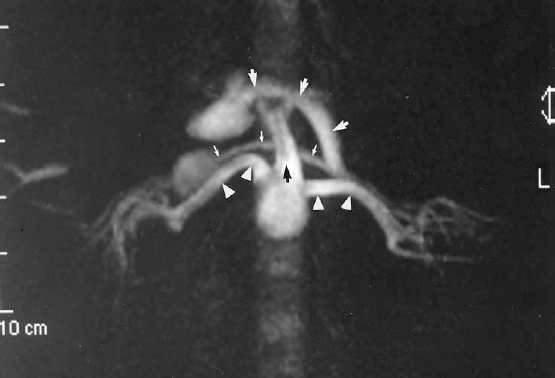
FIGURE 23-4. Magnetic resonance angiogram (MRA) of normal proximal renal arteries (white arrowheads). Axial maximum intensity projection (MIP) constructed from a three-dimensional phase-contrast intravenous MRA using gadolinium. Other structures depicted on this MIP are the superior mesenteric artery (SMA) (black arrow), left renal vein joining the inferior vena cava (IVC) (small white arrows), and splenic vein joining the portal vein (large white arrows) .
Contrast arteriography
Contrast arteriography is still the gold standard when it comes to anatomic detection of a renal artery stenosis; however, finding a renal artery stenosis in a hypertensive patient does not necessarily mean that the patient has renovascular hypertension. The renal artery arteriosclerotic lesion may be a consequence of the long-standing essential hypertension rather than a cause of it, or it may be totally unrelated. Furthermore, a significant percentage of patients who were found to have a renal artery stenosis at autopsy had been normotensive.13
Renal arteriography technique
When performing renal arteriography, a nonselective aortogram almost always is obtained first. The aortogram discloses the number of renal arteries supplying each kidney, the relative location of the renal arteries and kidneys, the origins of the renal arteries, and the aorta. When considering intervention for renovascular hypertension, the aortogram should be tailored to assess the celiac artery and its branches; in the event that surgical bypass is needed for treatment, these arteries frequently are used for inflow. The information on the aortogram facilitates the performance of selective renal arteriography and interventions, because without it, locating the renal artery orifice for selection may be more difficult and time consuming, accessory renal arteries may be missed, and renal artery origin disease may go undetected by a more distal selective catheter injection. Indeed, selective renal arteriography may not be needed in the evaluation of suspected renovascular hypertension if the aortogram clearly discloses a proximal renal artery stenosis or renal artery occlusion.
If the aortogram fails to disclose a lesion in a patient in whom there is a high suspicion of renovascular hypertension based on clinical history and noninvasive studies, selective renal arteriography must be performed. Transcatheter pressure gradient measurements can be helpful. Branch artery stenosis can be the cause of renovascular hypertension especially in children, and can be difficult to detect on nonselective aortography. Renovascular hypertension secondary to a distal branch artery stenosis can occur in FMD and less commonly in atherosclerotic disease. Specifics regarding technique and performance of aortography, selective vessel catheterization, angiographic contrast injections, and image acquisition are discussed in Chapter 2.
If arteriography discloses a significant renal artery stenosis, corroborated by clinical history, laboratory findings, and noninvasive studies, percutaneous transcatheter therapy can be initiated in the same session in selected patients.
Renal vein renin assay
Renal vein renin assay directly measures and compares the renin output of each kidney. It reflects the pathophysiology of renovascular hypertension. Combined with noninvasive modalities or angiographic detection of renal artery stenosis, renal vein renin assay increases the likelihood of confirming the presence of a lesion whose treatment will cure or improve the hypertension.
Blood samples are drawn from each renal vein and from the inferior vena cava below and above the renal veins. Care should be taken to ascertain whether any accessory renal veins are in the area. Also, whenever possible, the left renal vein sample is drawn peripheral to the gonadal vein to minimize dilution by nonrenal blood. Usually, a cobra-shaped catheter is used to select the renal veins. Peripheral selection of the left renal vein can be facilitated by the use of a tip deflector wire when necessary. To minimize the risk of blood aspiration impedance from the catheter tip invaginating the vein wall, a single side hole is punched in the catheter within 1 to 2 mm of the tip. Contrast injection is minimized because it can adversely affect the renin output measurements. Before blood samples are drawn, approximately 3 to 4 mL of blood in the catheter should be aspirated and discarded. Then approximately 5 mL of blood is slowly aspirated (to avoid causing excess turbulence in the blood flow during aspiration), and samples are placed on ice immediately to preserve them on route to the radioimmunoassay laboratory. Each sample must be meticulously labeled, and each sample location should be indicated on a vein diagram to allow exact correlation between assay levels and vein location.
The accuracy of renal vein renin sampling is diminished in patients with renal insufficiency, long-standing hypertension, and bilateral renal artery stenosis and also by the administration of antihypertensive medications during the assay. The ratio of renin levels of the two renal veins must be greater than 1.5:1 to be considered significant. A more complicated but more specific method for the use of renin levels in predicting the therapeutic response to the treatment of renal artery stenosis has been reported.14
Treatment
Before the advent of percutaneous transluminal balloon angioplasty (PTA), surgical bypass and endarterectomy were the only treatment options for renovascular hypertension. PTA has revolutionized the treatment of renal artery stenosis. Surgical bypass is still indicated in some patients with relative contraindications to renal PTA. Furthermore, surgical bypass is still an option for patients who fail to respond to renal PTA or who develop complications of PTA that are not correctable with percutaneous transcatheter measures.
In addition to the risk of general anesthesia, potential surgical morbidity includes renal failure, with a significant risk of cholesterol emboli related to cross clamping of a severely calcified atherosclerotically diseased aorta. The perioperative mortality rate is 2 to 4%.15 Successful surgical reconstructions have a somewhat better long-term primary patency than renal PTA, but the far greater morbidity and mortality of operative repair mitigate in favor of using PTA as the initial treatment, with surgery reserved for those patients who fail renal PTA or who have relatively strong contraindications to PTA.16
Percutaneous transluminal angioplasty
Pharmacologic aspects
Because of the chance of therapeutic benefit of post-renal PTA with a resultant precipitous drop in blood pressure, the patient’s antihypertensive medications must be appropriately adjusted. Long-acting antihypertensive medication must be discontinued prior to renal PTA; short-acting drugs (e.g., intravenous nitroprusside) should be substituted. One must be prepared to administer vigorous intravenous hydration to increase vascular volume in the event of a significant post-renal PTA blood pressure drop.
Risks of renal PTA include thrombosis and small artery branch spasm induced by catheter-guidewire manipulations. The risk of vasospasm can be minimized by administering an antispamodic drug at the outset of the procedure, such as a calcium channel blocker (e.g., nifedipine 10 mL sublingual). It is also essential to heparinize the patient therapeutically to minimize the risk of thrombosis. Doses for heparin range from 3,000 to 10,000 units, and adequacy of anticoagulation can be monitored by obtaining activated clotting times in the angiography suite. Low-dose aspirin (325 mg/day), beginning the day before and continuing for 6 months following PTA, also is advised by most practitioners to decrease the risk of post-PTA thrombosis and possibly the risk of restenosis.
Technique
After suitable angiographic evaluation of the renal artery stenosis, the affected renal artery is selected. Renal artery PTA necessitatesthe advancement of a guidewire well into the periphery of the renal arterial tree to ensure secure access across the stenosis for balloon advancement, which usually can be accomplished by using conventional selective catheters such as the cobra or simple C-shaped curve. In situations where the affected renal artery possesses a caudal angulation or where there is a very tight stenosis, conventional selective catheter shapes may not permit guidewire advancement across the stenosis or sufficiently peripheral guidewire advancement to allow catheter exchanges and balloon placement. In such a situation, use of a downward curving catheter such as a sidewinder, Simmons, or “shepherd’s crook” shape often can facilitate superselective secure catheterization. The advantage of the sidewinder curve in this circumstance is that withdrawing the catheter at the femoral puncture site will advance the catheter tip across the tight stenosis or into the downward angling renal artery with a force that is not obtainable with conventional cobra or simple C-shaped catheters.17,18 To minimize the risk of arterial dissection, it is important that catheter advancement across the renal artery stenosis be performed using a soft-tipped guidewire such as a Bentson (Cook Incorporated, Bloomington, IN) or TAD II (Mallinckrodt, St. Louis, MO) or during simultaneous contrast injection that will keep the tip off of the wall. A less commonly used approach for downward angling renal arteries is the axillary puncture.
After a catheter has been advanced securely into the renal artery, a guidewire must be advanced sufficiently peripherally into the renal arterial tree to permit safe catheter exchange and balloon advancement. The ideal guidewire has a short, floppy atraumatic end with rapid transition to a robust stiff section. The short soft tip is important to minimize the risk of trauma in the renal artery branch in which the wire is anchored, but it is important that the section of wire bridging the stenosis be stiff enough to guide the balloon successfully. A commonly used wire is the Rosen wire, which possesses these properties. There is controversy as to whether the guide-wire tip should possess a tight 1.5 mm radius “J” or be straight. The advantage of the “J” tip is that the tip presents a blunt end that is less likely to perforate a peripheral renal artery branch. The disadvantage of the “J” tip wire is that when it is wedged into an artery branch smaller in diameter than the radius of the “J,” it can induce vasospasm, a problem that is avoided when using a straight tip.
Intraarterial nitroglycerine should be administered immediately prior to superselective catheterization of the peripheral renal artery branches to minimize the risk of vasospasm. Nitroglycerine is effective and has a short half-life, allowing for administration of repeat doses (50 to 200 μg).
After the guidewire has been advanced into a peripheral renal artery branch, the balloon is advanced across the stenosis (Fig. 23-5). Balloon size is chosen based on the measured size of the adjacent “normal” renal artery on the angiogram. The balloon used for renal PTA should have a short length and short distance between the balloon’s distal end and the catheter tip. This configuration will minimize “torquing” of the renal artery with respect to the aorta as the balloon inflates and tries to straighten. Other catheter guidewire combinations that can be used to facilitate successful treatment of difficult renal lesions include hydrophilic balloon catheters to decrease friction, miniature low-profile balloon catheters such as the 3.8 Fr Medi Tech sub-4 balloons, and a balloon mounted on a guidewire (Teg wire, Medi Tech Inc.). The performance of renal PTA also can be facilitated by using an outer guiding catheter positioned at the renal artery orifice through which the balloon is advanced, allowing test injection of contrast with the balloon or guidewire still across the stenosis.
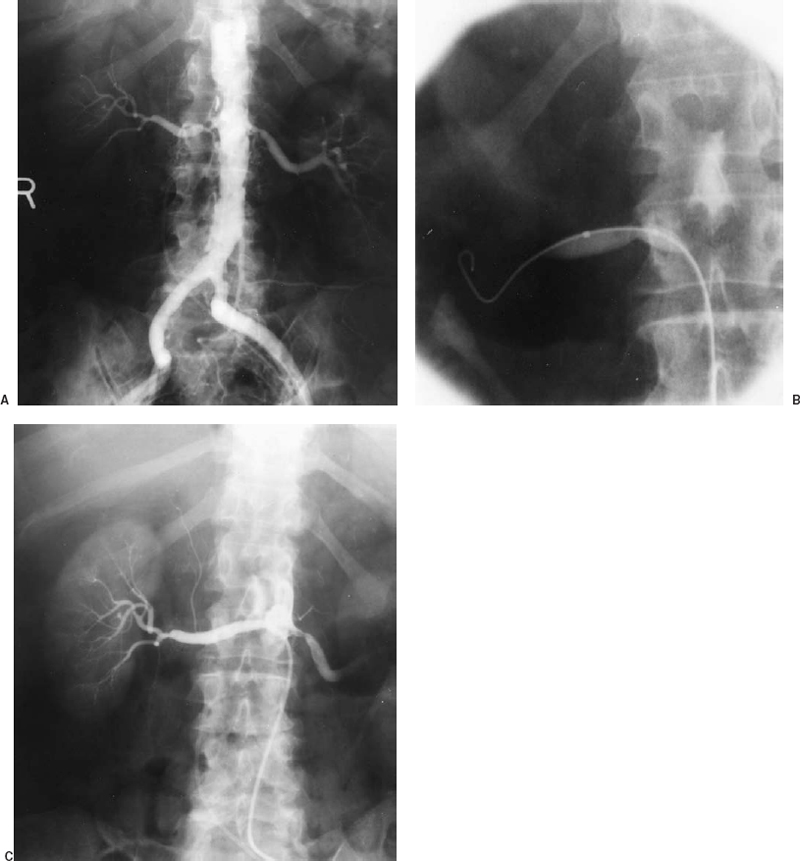
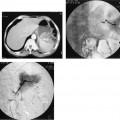
FIGURE 23-5. Renal artery angioplasty. A: Aortogram showing severe proximal right renal artery atherosclerotic stenosis (same patient as in Fig. 23-1) before angioplasty. B: Balloon being inflated across stenosis (note waist). C: Right renal arteriogram after angioplasty using sidehole catheter demonstrating good lumen response to angioplasty.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree