7
Vascular Recanalization Techniques
This chapter is an overview of the techniques of percutaneous intervention for recanalizing stenotic or occluded vessels. The indications and results for peripheral vascular, renal vascular, and venous intervention are discussed in subsequent chapters.
As early as 1964, Dotter and Judkins reported on the use of coaxial catheters to restore flow to a gangrenous extremity.1 In 1974, Gruentzig and Hopff introduced a double-lumen balloon catheter that revolutionized the field of interventional radiology.2 Stents, which were introduced in the late 1980s, helped to improve the results obtained with angioplasty alone. A suboptimal angioplasty now can be converted to a successful procedure with the placement of a stent. Acutely thrombosed vessels can be treated with the judicious use of thrombolytic agents. Lesions uncovered after clot dissolution can be treated with balloon angioplasty. These recanalization techniques can be used immediately after the diagnostic arteriogram either via the same percutaneous access used for the diagnostic arteriogram or through a second access site, thus simplifying the procedure.
Diagnostic arteriography should be performed only after a complete vascular workup, including noninvasive testing. Patients should have clear indications for revascularization, which include rest pain, nonhealing ulcers, gangrene, or severe claudication failing attempts at conservative therapy (i.e., smoking cessation and a consistent exercise program).3–5
When treating patients with peripheral vascular disease, we choose to access the vascular system, when possible, through the common femoral artery contralateral to the symptomatic extremity. This approach facilitates angioplasty or thrombolysis using the original puncture and does not preclude performing a second percutaneous puncture of the symptomatic extremity once the pathology is defined. The Seldinger technique, using either a double-wall or single-wall needle, is used to enter the common femoral artery. It is extremely important to enter the common femoral artery below the inguinal ligament and above its bifurcation to limit complications such as retroperitoneal hematomas, pseudoaneurysms, and arteriovenous fistulae.6,7 Using fluoroscopy, the common femoral artery is entered in its course over the lower half of the femoral head. The inferior epigastric artery and the deep circumflex iliac artery are good anatomic landmarks for the position of the inguinal ligament because these vessels arise off of the most distal aspect of the external iliac artery. If a prior angiogram is available, it should be reviewed to note the position of these vessels. It is safe to assume, however, that the inguinal ligament does not dip below the midfemoral head in almost all cases. It is important to puncture the common femoral artery over the midfemoral head. Too high a puncture may lead to retroperitoneal hematomas secondary to an inability to achieve adequate compression, and too low a puncture may lead to an arteriovenous fistula because the artery and vein tend to overlie each other in this region.
When treating peripheral vascular disease, a bilateral femoral arteriogram is performed from the level of the renal arteries to the feet. Appropriate oblique views of the pelvis and groin must be obtained to exclude lesions at the bifurcations of the common iliac artery and common femoral artery. When evaluating the angiogram, one must take into consideration the patient’s history, physical examination, and noninvasive studies. If the angiogram does not correlate with these studies, additional views must be obtained to uncover lesions that may not be clearly evident on the original arteriogram.
Percutaneous transluminal angioplasty for peripheral atherosclerotic disease is best performed on short focal stenoses or occlusions in large vessels. Lesions longer than 10 cm are considered less likely to have good initial and long-term results.8,9 With the use of stents, however, even long lesions in the aortoiliac distribution can be treated successfully.10 Results in the femoropopliteal distribution are less rewarding; however, when dealing with shorter lesions, results are acceptable. Even tibial vessels can be treated with angioplasty; however, these vessels should be treated only for limb-salvage indications. When treating occlusions, one must be convinced that the occlusion is chronic. If the occlusion is relatively acute, the incidence of embolization resulting from a clot may be high. Therefore, if an occlusion is suspected to be less than 6 weeks old, a primary angioplasty without prior thrombolysis probably should not be performed.
 Aortoiliac Angioplasty
Aortoiliac Angioplasty
If an aortic or iliac lesion is identified or suspected, pressures must be obtained above and below the lesion to document its hemodynamic significance. Any resting gradient is considered significant. If no resting gradient is present, a vasodilator such as 60 mg of papaverine or 100 to 200 μg of nitroglycerin should be administered intraarterially distal to the lesion in question. A peak systolic gradient of greater than 10 mm of mercury is considered significant.11 When the outflow is markedly obstructed (such as in the presence of both superficial femoral artery and profunda femoral artery disease), there may not be a demonstrable gradient, despite the presence of a high-grade stenosis. These lesions should be identified and treated. If a bypass is performed below a significant lesion, the bypass may be compromised as a result of inadequate flow. Iliac angioplasty and stent placement can be performed around the aortic bifurcation using the puncture site of the diagnostic arteriogram. In many circumstances, we choose to puncture the ipsilateral common femoral artery in a retrograde fashion. This approach allows for simultaneous arterial pressure measurements above and below the lesion and facilitates accurate straight-line stent placement at the origin of the common iliac artery, if necessary (Fig. 7-1).
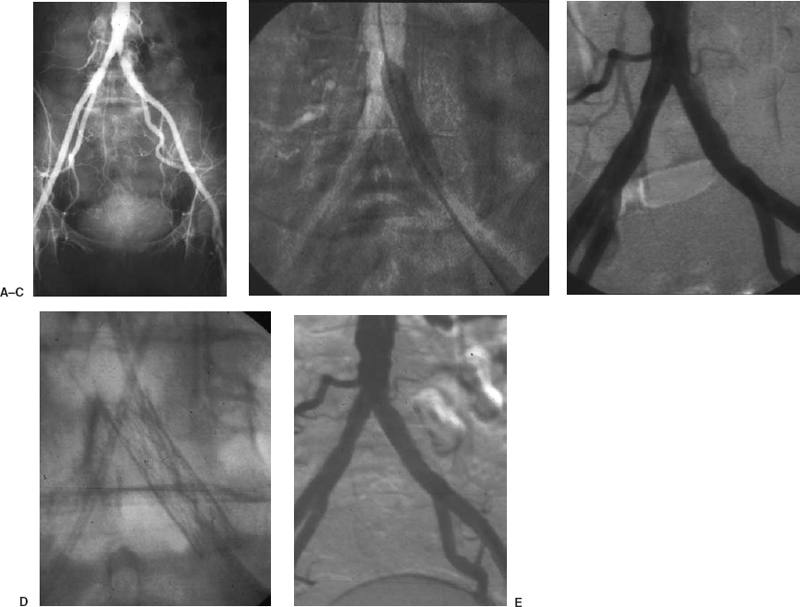
FIGURE 7-1. A 45-year-old man, a smoker presented with half block claudication on the left. (A) Angiogram via the right common femoral artery demonstrates a focal moderate to severe stenosis at the origin of the left common iliac artery. A left common femoral artery puncture is performed, and pressures obtained above and below the lesion demonstrate a 40-mm mercury gradient. (B) Percutaneous angioplasty is performed with an 8 mm × 4 cm balloon via the left common femoral artery approach. (C) Postpercutaneous transluminal angiography demonstrates a residual defect with persistent pressure gradient. (D) A Palmaz P294 stent (Cordis, Johnson & Johnson Corp.,) is deployed across the lesion. (E) Poststenting angiogram demonstrates resolution of the lesion. No further pressure gradient is observed.
All interventions should be performed through a vascular sheath. The sheath usually facilitates the intervention by allowing for rapid catheter exchange, easier post-procedure angiographic evaluation, and decreased patient discomfort in the groin; it is associated with a lower incidence of complications. All patients are routinely pretreated with aspirin as an antiplatelet agent. Although many physicians use intraprocedural anticoagulation with heparin, its value is unconfirmed, and excessive use of heparin may lead to a higher incidence of local complications such as hematomas and pseudoaneurysms. With the current advances of endoluminal closure devices, full anticoagulation is less problematic. Ideally, tight stenotic lesions should be crossed using road mapping. The best guidewire catheter combination to cross the stenosis depends on the lesion and the operator.
Long lesions, external iliac artery lesions, and occlusions also can be treated. The optimal percutaneous therapy for iliac occlusions has not yet been determined. These lesions have been treated with initial lysis and subsequent angioplasty or stenting of the underlying lesions. Other successful treatments include primary stenting of iliac occlusions12–15 (Fig. 7-2). The response to balloon angioplasty of longer lesions is often less than ideal. Suboptimal angioplasty or an occlusive dissection is not uncommon; therefore, many interventional radiologists would recommend stenting these lesions primarily (Fig. 7-3). A balloon-expandable stent (e.g., Palmaz-Schatz, Cordis, Johnson & Johnson Corp., New Brunswick, NJ, U.S.A.) (see Fig. 7-1B) an IntraStent (Intratherapeutics, St. Paul, MN, U.S.A.) (seeFig. 7-3) or a self-expanding stent such as the Wallstent (Boston Scientific Corp., San Ramon, CA, U.S.A) or Smart Stent (see Fig. 7-17D) (Cordis, Johnson & Johnson Corp., New Brunswick, NJ, U.S.A.) (see Fig. 7-17D) can be placed. Suboptimal angioplasty of ideal lesions also can be managed by placing a stent (see Fig. 7-1). After intervention, pressures should be obtained again to assess hemodynamically the adequacy of the intervention.11,16–18
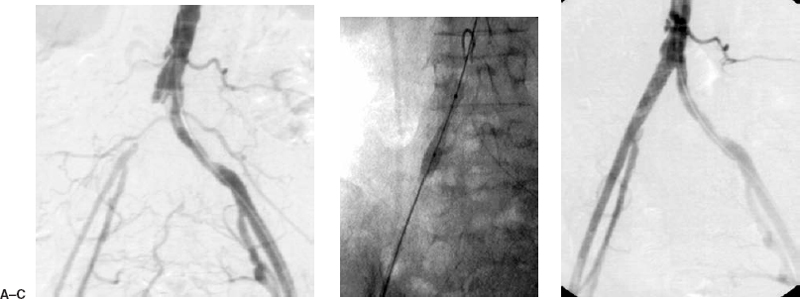
FIGURE 7-2. An 82-year-old woman presented with severe claudication of the right lower extremity. (A) Diagnostic arteriogram from the left common femoral artery demonstrates an occlusion of the right common iliac artery. (B) A retrograde puncture of the right common femoral artery was performed, and a guidewire was passed across the lesion. The lesion was primarily stented with a Palmaz P394 stent (Cordis, Johnson & Johnson Corp.). (C) Postangioplasty and stenting angiogram demonstrates the recanalized right common iliac artery. Note that the right external iliac artery has an enlarged caliber as a result of increased arterial flow.
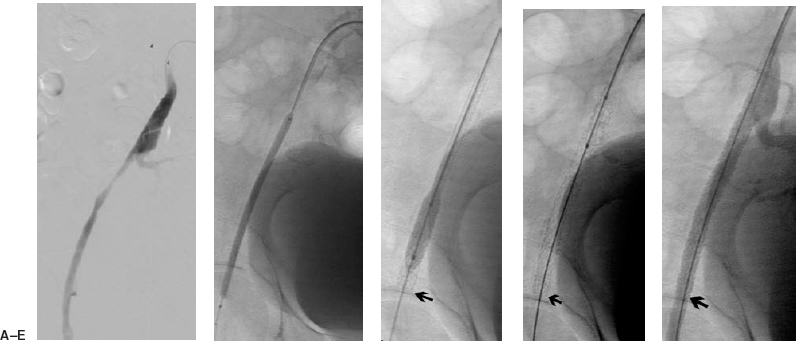
FIGURE 7-3. A 78-year-old man presented with a failing right femoral to distal bypass graft. (A) Initial arteriogram was performed from the left common femoral artery, a Sos Omni flush catheter (Angiodynamics) was advanced into the right common iliac artery, where a selective right iliac angiogram demonstrates a long external iliac stenosis. (B) An up and over Balkin contralateral 7 Fr sheath (Cook Group Company) was advanced into the right common iliac artery from the initial left common femoral artery puncture. Aguidewire is advanced beyond the lesion and a 4 mm × 10 cm balloon was used to deliver a 78-mm-long IntraStent (Intratherapeutics) across the lesion. (C) The stent is opened further with a 7 mm × 2 cm balloon. (D) There is no foreshortening with this balloon-expandable stent. (E) Poststenting angiogram demonstrates resolution of the lesion.
Most complications of angioplasty can be managed nonoperatively. The most common complication is a hematoma, usually self-limited at the puncture site. Pseudoaneurysms can be treated definitively by using ultrasound-guided compression or percutaneous thrombin injection. In situ thrombosis or distal embolization during or after angioplasty can be treated with intraarterial thrombolytics, with suction thromboembolectomy using a guiding catheter (Fig. 7-4), or with the Possis Angiojet device (Possis Medical, Minneapolis, MN, U.S.A.). Obstructing flaps can be stented. Iliac rupture is a feared complication because these patients may require surgery to prevent exsanguination. Iliac rupture is suggested by the presence of continued pain after the angioplasty balloon is deflated and the presence of free extravasation of contrast after angioplasty. The angioplasty balloon should be immediately reinflated across the lesion to tamponade the rupture. The patient then can be considered for transfer to the operating room for repair of the vessel; alternatively, a covered stent (if available) can be delivered via the femoral access to exclude the rupture (Fig. 7-6).
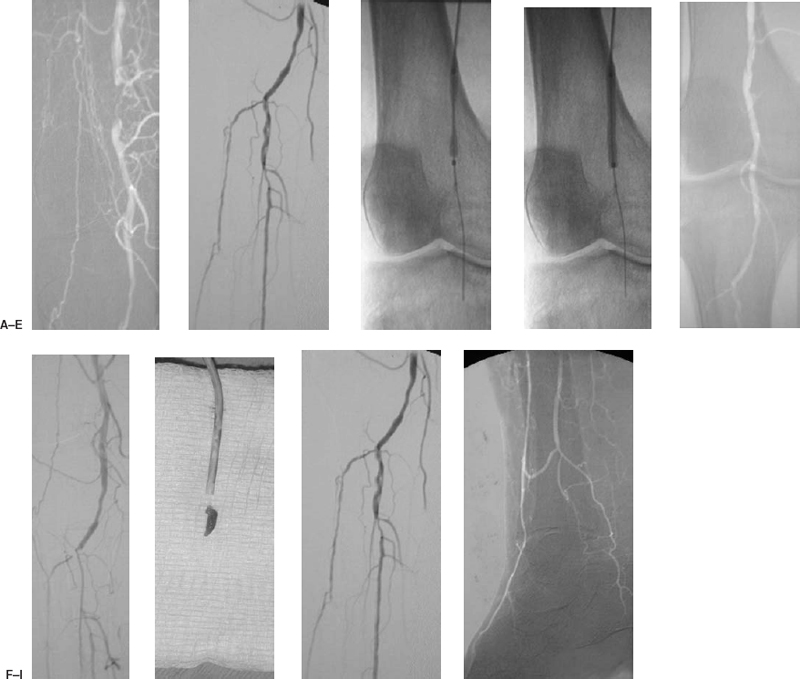
FIGURE 7-4. An 83-year-old man with diabetes presented with a nonhealing ulcer of the right heel. (A) A short-segment occlusion of the popliteal artery is identified on the initial arteriogram performed via the left common femoral artery. A right common femoral antegrade puncture is performed to treat the lesion, and this digital image of the lesion is acquired. (B) Arteriogram of the distal runoff demonstrates occlusions of the midportion of the anterior tibial and of the posterior tibial arteries. The tibialperoneal trunk is patent, and there is mild disease at the origin of the peroneal artery. (C) Angioplasty of the popliteal lesion is performed with a 4 mm × 4 cm balloon. (D) The balloon is brought to full profile. (E) Postangioplasty angiogram demonstrates resolution of the popliteal occlusion, but the distal runoff appears obstructed. (F) Occlusion of the anterior tibial artery and tibialperoneal trunk is noted in the runoff evaluation. A 6 Fr guiding catheter is advanced over the guidewire to the site of occlusion. (G) Aspiration thromboembolectomy is performed with the guiding catheter, and the embolus is removed. The catheter and aspirated embolus are displayed on the gauze pad. (H and I) Postaspiration angiogram demonstrates recovery of the original runoff.
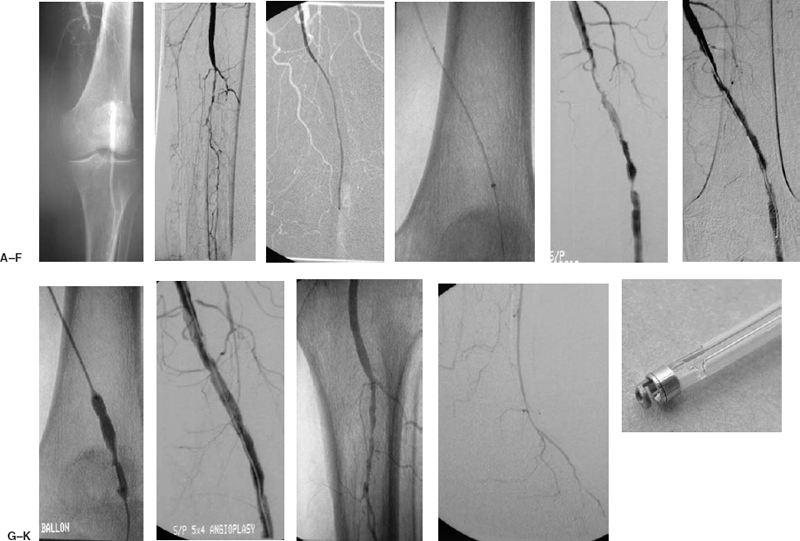
FIGURE 7-5. A 68-year-old man presented with new-onset rest pain in the right lower extremity. (A) Diagnostic angiogram is performed from the right common femoral artery, demonstrating occlusion of the left popliteal artery. (B) Significant three-vessel tibial disease is noted with reconstitution of the distal anterior tibial artery. (C) An up and over Balkin contralateral 5.5 Fr sheath (Cook Group Company) was advanced into the external iliac artery. A 5 Fr Berenstein catheter (Angiodynamics) was advanced over a Bentson guidewire (Angiodynamics, Inc.) into the popliteal artery, and a Possis Angiojet device (Possis Medical) was passed over a V-18 control wire (Boston Scientific) across the occlusion. (D) Closeup of the Possis Angiojet removing thrombus. (E) Post-Possis angiogram demonstrates a channel and underlying lesions. At this point, the patient became asymptomatic. Two hours of thrombolysis with rt-PA was performed at 2 mg/hour drip into the superficial femoral artery to dissolve any residual clot that may not have been removed by the Possis device. (F) Significant improvement was noted following thrombolysis. (G) An angioplasty of the stenotic lesion was performed with a 5 mm × 4 cm balloon. (H) Postangioplasty angiogram demonstrates a good result. (I) The infrapopliteal angiogram is unchanged. (J) There is reconstitution of the distal anterior tibial and dorsalis pedis arteries. (K) Photograph of the Possis Angiojet’s Halo catheter.
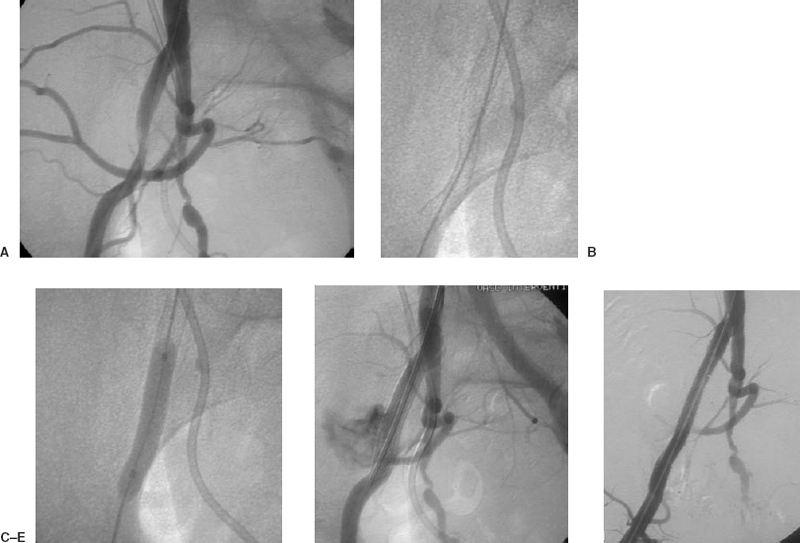
FIGURE 7-6. A 57-year-old woman with metastatic cervical cancer, postradiation therapy, presented with claudication progressing to rest pain in the right lower extremity. (A) Diagnostic arteriogram performed from the right common femoral artery demonstrates a significant lesion across the right external iliac artery. A significant pressure gradient is identified. (B) A 7 × 60 mm Smart Stent (Cordis, Johnson & Johnson Corp.) is deployed across the lesion. Following angioplasty, rupture of the iliac artery is suspected from the patient’s continued pain and confirmed with angiography. (C) The balloon is inflated across the rupture to control the bleeding. (D) Bleeding continues despite prolonged balloon inflation. (E) A covered stent is deployed, the rupture is controlled (as demonstrated in the poststenting angiogram), and the iliac lesion is resolved.
 Superficial Femoral and Popliteal Artery Intervention
Superficial Femoral and Popliteal Artery Intervention
Stenoses or occlusions of the superficial femoral artery and popliteal artery up to 10 cm long are considered amenable to balloon angioplasty. These lesions can be approached from the contralateral extremity by using the common femoral artery access created during the diagnostic arteriogram (Fig. 7-7). Using an “over-the-corner” sheath markedly facilitates the advancement of balloon catheters across lesions using the contralateral approach.
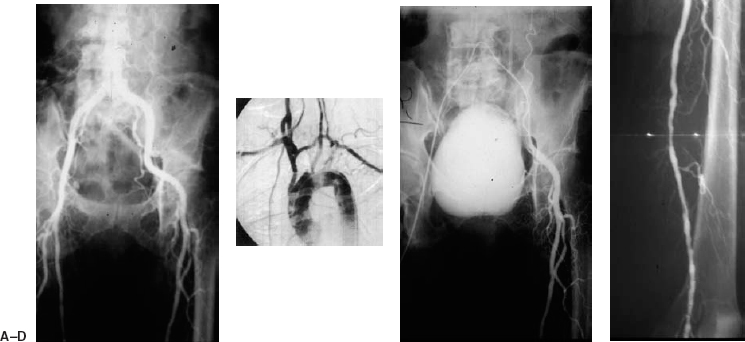
FIGURE 7-7. A 70-year-old woman, a smoker with diabetes, presented with a nonhealing ulcer of the left foot. (A) Long-leg cut-film film angiogram demonstrates a steep bifurcation of the aorta. (B) Mild to moderate disease is present in the superficial femoral artery and popliteal artery, with a severe (99%) stenosis of the proximal popliteal artery in the adductor canal. (C
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree