History and Clinical Findings
A 36-year-old woman presented at the Neurology Clinic complaining of dizziness that had begun 8 days earlier following a chiropractic manipulation of her cervical spine. Three days earlier she had been hospitalized with a hyper-tensive episode and had been diagnosed clinically with a suspected vertebral artery dissection. Neurologic examination revealed minimal anisocoria (left < right), mild bradydysdiadochokinesia, slight unsteadiness in stance and gait testing, and hypoesthesia of the radial left fingers and left forearm. Duplex ultrasound and MRI excluded a vascular dissection. MRI showed rounded hyperintensi-ties several millimeters in size distributed in the white matter of both hemispheres and extending to the subcortical level. Microangiopathic changes were considered to be the most likely cause (Fig. 7.1). Because the patient had a long known history of asymptomatic Crohn disease, the differential diagnosis also included vasculitis. Laboratory serum and liquor inflammatory markers were not significantly elevated. MR angiography showed long segmental narrowing of the right vertebral artery, predominantly affecting Berguer segments 3 and 4, as a normal variant. The patient was hospitalized. Four days later she was referred to radiology for diagnostic angiography of the cerebral vessels. The clinical question was: High index of suspicion for cerebral vasculitis in Crohn disease, angiography before corticosteroid therapy, vasculitic changes? Informed consent on the day before angiography included disclosure of the following potential complications: allergy, shock, death, bleeding, emergency surgery, vascular injury, vascular dissection, paraplegia, thrombosis, embolism, stroke, and possible need for long-term care. When questioned, the patient denied having any allergies.
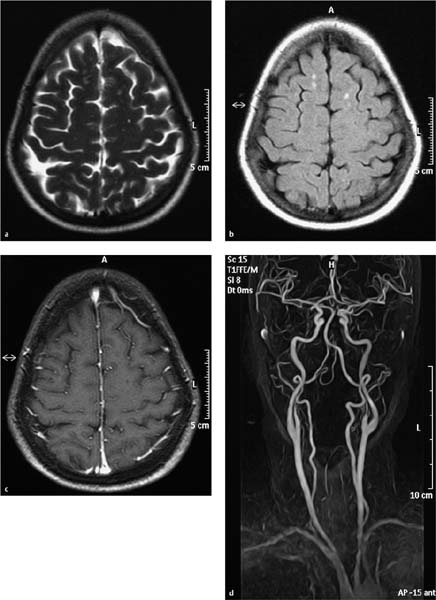
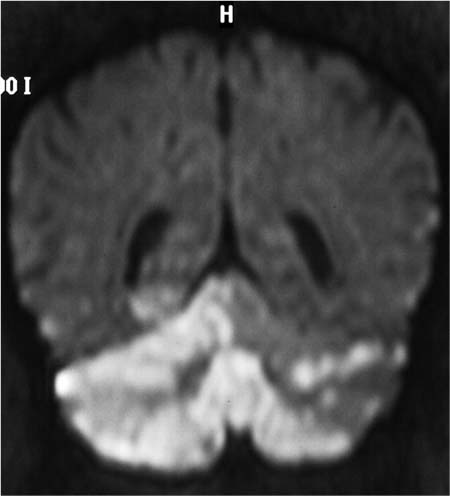
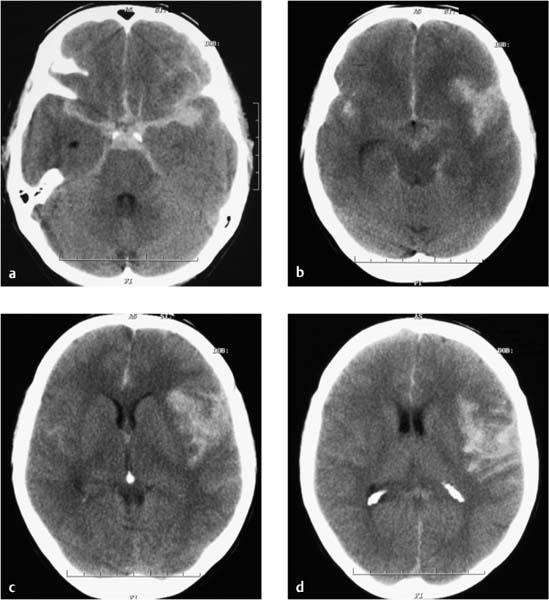
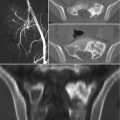
Fig. 7.1a–d MRI on the day of hospital admission. Rounded, bilateral white-matter lesions up to approximately 5 mm in size have low T1-weighted signal intensity and high signal intensity on T2-weighted and FLAIR images (a, b), and do not enhance after contrast administration (c). They were considered most likely to have a postischemic or vasculitic cause. Decreased calibers in the right vertebral artery are a normal variant and are most apparent in segments 3 and 4 (d).
a T2-weighted image without contrast medium.
b Flair-weighted image without contrast medium.
c T1-weighted image after IV contrast administration.
d MR angiographic sequence.
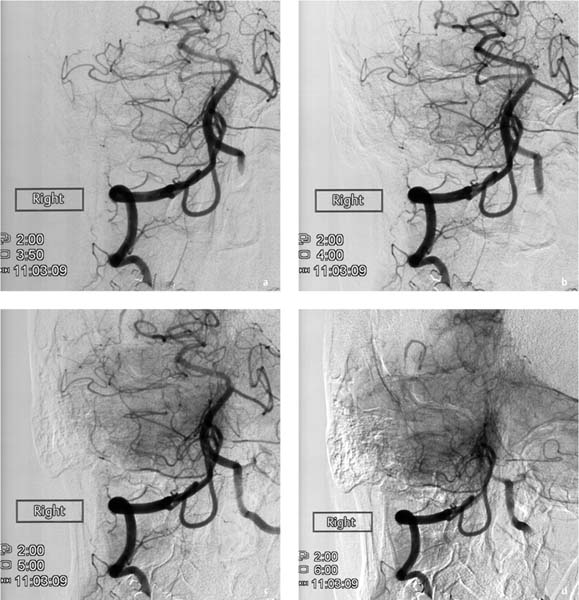
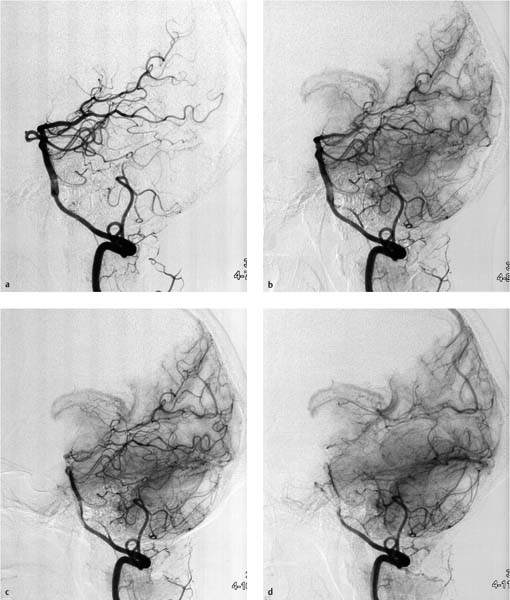
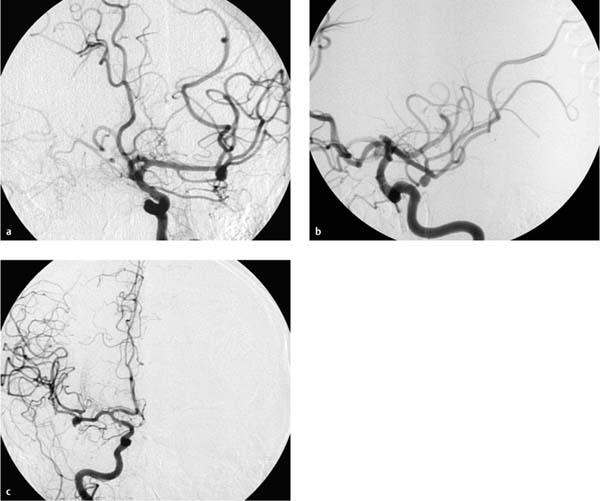
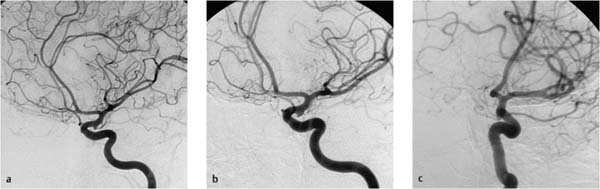
Fig. 7.2a–e Chronologically first angiographic series in the sagittal projection, taken at 11:03. Representative preexisting segmental images in the early arterial phase (a), late arterial phase (b), early venous phase (c), and late venous phase (d). The series confirms hypoplasia of the right vertebral artery and a contrast stasis in the right vertebral artery..
a Chronologically first image documented in the series.
b Chronologically second image documented in the series.
c Chronologically third image documented in the series.
d Chronologically fourth image documented in the series.
e Chronologically fifth image documented in the series.
The angiographic study was performed by a fourth-year radiology resident who had 8 months’ clinical experience in performing diagnostic angiography. He was assisted by a third-year radiology resident. A supervising physician and the department head were standing by in case unexpected problems arose. Despite oral premedication with 1 mg lorazepam, the patient appeared anxious before and during the examination. A Teflon guidewire was placed in the right femoral artery using Seldinger technique. A 5 French (1.67 mm) sheath was introduced over the guidewire, and then a 5 French catheter with a vertebral artery configuration (Cordis) was advanced into the suprarenal aorta. In that position the hydrophilically coated guidewire with a soft, curved tip was inserted (Terumo Europe NV). The vertebral artery catheter was introduced over the Terumo guidewire and advanced into the ascending aorta under fluoroscopic guidance. Next the guidewire was withdrawn into the catheter. Still under fluoroscopic guidance, the catheter was inverted so that it could be maneuvered into the supra-aortic vessels. The catheter was carefully withdrawn under fluoroscopic control until it entered the origin of a supra-aortic artery. Contrast injection under fluoroscopic control confirmed that the tip of the catheter was in the brachiocephalic trunk. From that position the Terumo guidewire was advanced into a cervical vascular branch. The guidewire was removed. Catheter placement was checked by manual injection of 2–3 mL of contrast medium (iohexol, Accupaque, GE Healthcare, concentration 300 mg/mL), which confirmed that the right vertebral artery had been catheterized. At that moment the patient complained of severe nausea. She turned her head to the left side and vomited several times. Both radiologists interpreted this event as an allergic reaction to the iodinated contrast medium. The catheter was withdrawn into the aortic arch, whereupon 50 mg of Ranitic (ranitidine, Hexal), 4 mg of Fenistil (dimethindene maleate, Novartis), 250 mg of Prednisolut (prednisolone-21-hydrogen succinate, Mibe GmbH), and 1 ampoule of Vomex (62 mg, dimenhydrinate, Astellas Pharma) were administered intravenously. Several minutes later the patient’s clinical status had improved, and the right vertebral artery was again catheterized. Fluoroscopically guided contrast injection confirmed correct catheter placement with the tip approximately 2 cm cranial to the origin of the vertebral artery. As indicated by the electronic clock of the angiography system (Allura Xper FD 20, Philips Healthcare), the first series of sagittal angiographic images was taken at 11:03 following the manual injection of 6 mL undiluted contrast medium (Fig. 7.2). A second, lateral series was obtained at 11:06. Because the patient had moved during acquisition of the second series, the sequence was repeated at 11:07 (Fig. 7.3). At that point in the examination, the patient became unresponsive to verbal commands. Inspection revealed ptosis of the right eye.
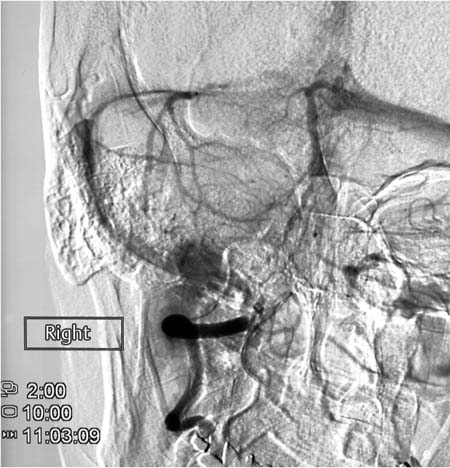
Fig. 7.3a–d Chronologically third angiographic series in the lateral projection, taken at 11:07.
a Chronologically first image documented in the series.
b Chronologically second image documented in the series.
c Chronologically third image documented in the series.
d Chronologically fourth image documented in the series.
Further Case Summary
When neurologic symptoms arose, the chief radiologist entered the room and terminated the examination. The catheter and sheath were removed. The supervising neurologist was summoned by telephone to the angiography suite. The catheter and sheath were removed. The puncture site in the right femoral artery was occluded with Angioseal STS Plus (St. Jude Medical). Scrutiny of both angio-graphic series showed that blood flow was already diminished during acquisition of the first image series. Initially the contrast bolus drained normally through the hypoplastic right vertebral artery (Fig. 7.2a), but later in the series the caliber of the vessel decreased and perfusion was delayed. By the end of the series, some opacified blood was still present in the right vertebral artery (Fig. 7.2e). Filling defects were not seen initially, suggesting that a vasospasm was the most likely diagnosis.
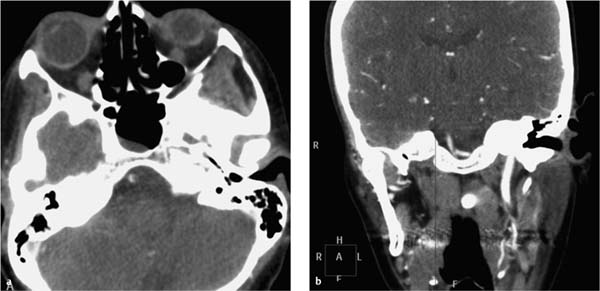
Subsequent CT angiography of the cerebral supply arteries (at 11:23) revealed segmental occlusions of both vertebral arteries and a thrombus at the junction of the vertebral arteries with the basilar artery (Fig. 7.4). A vasospasm with spasm-induced thrombus formation at the confluence of the vertebral arteries was still considered to be the most likely diagnosis. Management options were discussed with the senior neurologist and a neuroradiolo-gist. The consensus was to initiate intravenous thrombolytic therapy with Actilyse (alteplase, Boehringer Ingelheim) in order to avoid future thrombus formation induced by the spasm-induced disturbances of blood flow. Intra-arterial thrombolysis was withheld because by the time of the intra-arterial angiography, no thrombembolus had been visualized. The spasmolytic agent Nimodipin (nimodipine, Carinopharm) was also administered intravenously. The neurologist and neuroradiologist also recommended heparinization.
Fig. 7.4a, b CT angiography at 11:23 demonstrates a thrombus in the right vertebral artery and thrombotic occlusion of the left vertebral artery (a). A thrombus is also detected at the confluence of the vertebral arteries just proximal to the basilar artery (b).
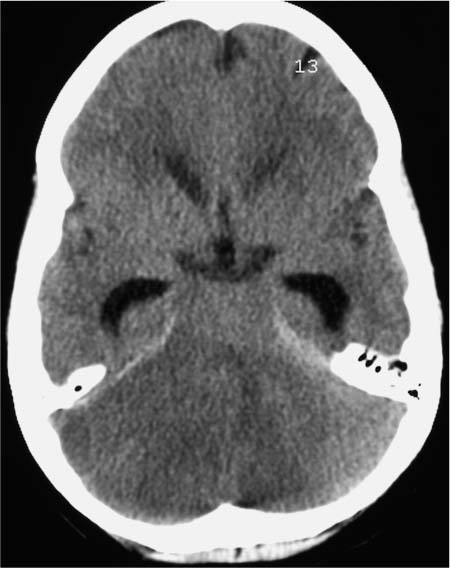
The patient was transferred to the stroke unit. MRI was performed 5 hours later (Fig. 7.5). Diffusion-weighted sequences showed ischemia-induced diffusion changes in both cerebellar hemispheres (right > left), in the cerebellar vermis, in the right mesencephalon, and in the territory of the right posterior cerebral artery. By this time both vertebral arteries were freely perfused. Long segmental hypoplasia of the right vertebral artery, predominantly affecting segments 3 and 4, was still present and was unchanged relative to preangiographic MRI. The white-matter lesions also appeared unchanged. Computed tomography confirmed a cerebellar infarction. Perifocal edema had led to the development of internal hydro-cephalus (Fig. 7.6). A decompressive occipital craniectomy was performed the same evening.
Three months after the intervention, the patient still suffered from predominantly left-sided spastic tetraparesis and aphasia. Her vigilance had improved. She was now able to sit up for 3 hours a day and could communicate with her environment through eye movements and grunts. Dysphagia and myoclonic spasms were regressive. While the attending neurologists believed that further improvement was likely, they agreed that the patient would have severe disabilities for the rest of her life.
Fig. 7.5 MRI at 16:15 shows ischemia-induced diffusion abnormalities in both cerebellar hemispheres, the vermis, the right mesencephalon, and the territory of the right posterior cerebral artery. At this time both vertebral arteries are freely perfused as proven by an MR-angiographic sequence not visualized.
Fig. 7.6 CT at 23:13 displays a fresh cerebellar infarction. Internal hydrocephalus has developed due to compression of the aqueduct by edema.
Error Analysis and Strategy for Error Prevention
The following errors were made during diagnostic angiography of the cerebral arteries related to the indication for performing the examination, the examination technique that was used, and misinterpretation of the symptoms that arose during angiography, and the management of the complicaton.
 There was only a relative medical indication for intra-arterial DSA of the cerebral arteries. It is true, of course, that conventional angiography is better than magnetic resonance angiography for detecting slight caliber variations and microaneurysms of the small cerebral arteries in the setting of vasculitis because of its superior spatial and contrast resolution. But when it comes to patient selection, the potential therapeutic benefit of intra-arterial DSA should always be weighed against the facilities available in the particular situation and risk of complications.
There was only a relative medical indication for intra-arterial DSA of the cerebral arteries. It is true, of course, that conventional angiography is better than magnetic resonance angiography for detecting slight caliber variations and microaneurysms of the small cerebral arteries in the setting of vasculitis because of its superior spatial and contrast resolution. But when it comes to patient selection, the potential therapeutic benefit of intra-arterial DSA should always be weighed against the facilities available in the particular situation and risk of complications.
According to published data (review in Morris 2007), between 0.3% and 6.8% of patients who undergo cerebral arteriography develop new, sometimes transient neurologic symptoms referable to angiography within 72 hours after undergoing the procedure. Studies that included all patients undergoing angiography for suspected brain ischemia and all new neurologic symptoms put the rate of all neurologic sequelae at 9–12% and the rate of permanent neurologic deficits at 5%. According to the American College of Radiology (ACR), the reported rates of arterial occlusion requiring surgical thrombectomy or thrombolysis range between 0.0% and 0.4%, and the rates of reversible neurologic deficits and permanent neurologic deficits should not exceed 2.5% and 1.0%, respectively.
The patient in this case was a 36-year-old woman with mild neurologic symptoms that were noted only as incidental findings. Her Crohn disease had been asymptomatic for years. She was hospitalized for dizzy spells following a chiropractic manipulation of the cervical spine. Duplex ultrasound, MRI, and MR angiography had excluded a vertebral artery dissection as a possible complication of the treatment. While she was hospitalized, she experienced hypertensive episodes which, like the dizzy spells, were not causally related to the findings of the neurologic examination. The differential diagnosis of cerebral vasculitis was based on the white-matter lesions that had been detected incidentally by MRI, although the inflammatory serum and liquor parameters were not significantly elevated. Morphologically, the MRI changes were consistent with the sequelae of microangiopathy secondary to hypertensive disease or atherosclerosis. Given their distance from the corpus callosum, circumscribed nature, and subcortical location, they were not consistent with multiple sclerosis. Florid vasculitis was excluded by the lack of enhancement after IV contrast administration. Moreover, areas of cerebral vasculitis caused by a variety of pathogenic organisms and mechanisms tend to appear on MRI as larger enhancing areas with ill-defined margins. MR angiography had shown no evidence of vascular changes typical of vasculitis (stenoses, pseudoaneurysms of the small cerebral arteries). In retrospect, even a positive DSA result would not have justified corticosteroid therapy in this situation, and so the sole purpose of intra-arterial DSA was to confirm the diagnosis and not to direct therapeutic decisions.
 Recognized risk factors for complications are patient age > 70 years, the angiographic investigation of strokes or TIAs, the angiographic detection of ≥ 50% or ≥ 70% stenoses, procedure times longer than 60 minutes, the use of multiple catheters, and the experience of the radiologists/neuroradiologists in performing the examination. In a multicenter analysis of 5000 catheter cerebral angiographic procedures, the rate of severe permanent neurologic sequelae and deaths was found to be 3.9% in training hospitals and 0.9% in nontraining hospitals (Mani et al. 1978). Willinsky et al. (2003) obtained very similar results (1.3% vs. 0.5%) in their analysis of 2899 cerebral angiograms. For this reason, it would have been desirable for a specialist experienced in angiographic techniques to have been present from the start of the procedure in this patient. When the first neurologic symptoms appeared, the specialist could have quickly intervened and terminated the examination.
Recognized risk factors for complications are patient age > 70 years, the angiographic investigation of strokes or TIAs, the angiographic detection of ≥ 50% or ≥ 70% stenoses, procedure times longer than 60 minutes, the use of multiple catheters, and the experience of the radiologists/neuroradiologists in performing the examination. In a multicenter analysis of 5000 catheter cerebral angiographic procedures, the rate of severe permanent neurologic sequelae and deaths was found to be 3.9% in training hospitals and 0.9% in nontraining hospitals (Mani et al. 1978). Willinsky et al. (2003) obtained very similar results (1.3% vs. 0.5%) in their analysis of 2899 cerebral angiograms. For this reason, it would have been desirable for a specialist experienced in angiographic techniques to have been present from the start of the procedure in this patient. When the first neurologic symptoms appeared, the specialist could have quickly intervened and terminated the examination.
 From a procedural standpoint, the carotid arteries should have been catheterized first for anatomical reasons. Also, they supply brain areas that are less critical than those supplied by the vertebral arteries. Another factor is that, in the case presented here, the white-matter lesions requiring etiologic investigation were located in the territory of the anterior and middle cerebral arteries. Hence it would have been sufficient to detect or exclude vasculitic changes in the anterior circulation.
From a procedural standpoint, the carotid arteries should have been catheterized first for anatomical reasons. Also, they supply brain areas that are less critical than those supplied by the vertebral arteries. Another factor is that, in the case presented here, the white-matter lesions requiring etiologic investigation were located in the territory of the anterior and middle cerebral arteries. Hence it would have been sufficient to detect or exclude vasculitic changes in the anterior circulation.
 In view of the poor prognosis, intra-arterial thrombolytic therapy using rt-PA (recombinant tissue plasminogen activator) would have been warranted either directly after the onset of the neurologic symptoms or at least after the visualization of thrombotic material in both vertebral arteries in CT angiography carried out 20–30 minutes thereafter. According to current literature this would have been a more promising approach to achieve a quick re-vascularization than the intravenous thrombolytic therapy favored in this case. However, whether intra-arterial rt-PA-therapy would have prevented the unfortunate course remains unknown.
In view of the poor prognosis, intra-arterial thrombolytic therapy using rt-PA (recombinant tissue plasminogen activator) would have been warranted either directly after the onset of the neurologic symptoms or at least after the visualization of thrombotic material in both vertebral arteries in CT angiography carried out 20–30 minutes thereafter. According to current literature this would have been a more promising approach to achieve a quick re-vascularization than the intravenous thrombolytic therapy favored in this case. However, whether intra-arterial rt-PA-therapy would have prevented the unfortunate course remains unknown.
 In the present case the pathologic cause of the complication remains uncertain. Most likely it resulted from vasospasms induced by the intra-arterially applied contrast medium and/or the catheter manipulation as assumed during the complication management phase of the intervention, the thrombemboli CT angiographically visualized in both vertebral arteries 20–30 minutes after the onset of the symptoms being due to the disturbances of blood flow secondarily induced by the vasospasms. A systemic allergic reaction to intra-arterial contrast administration is another possible explanation for vasospastic reactions of the cerebral vasculature despite the high physiological tolerance of modern iodinated contrast media. A local vasomotor reaction of the vertebral artery to intra-arterial contrast administration is another possibility. Local vaso-motor reactions to intra-arterial contrast media were first described in the 1980s, mainly in association with cardiac catheterizations. With advances in the pharmacological development of contrast media, this type of complication is no longer reported in the literature and thus seems to berare in clinical routine. Thrombembolism induced by catheter manipulation is another possible cause. Lastly, a thrombus that had formed in the distal catheter lumen during the time when the guidewire had been withdrawn into the catheter and the distal lumen was occupied not by the guidewire but by stagnant blood might have been be embolized into the cerebral arteries by injection of contrast medium via the catheter.
In the present case the pathologic cause of the complication remains uncertain. Most likely it resulted from vasospasms induced by the intra-arterially applied contrast medium and/or the catheter manipulation as assumed during the complication management phase of the intervention, the thrombemboli CT angiographically visualized in both vertebral arteries 20–30 minutes after the onset of the symptoms being due to the disturbances of blood flow secondarily induced by the vasospasms. A systemic allergic reaction to intra-arterial contrast administration is another possible explanation for vasospastic reactions of the cerebral vasculature despite the high physiological tolerance of modern iodinated contrast media. A local vasomotor reaction of the vertebral artery to intra-arterial contrast administration is another possibility. Local vaso-motor reactions to intra-arterial contrast media were first described in the 1980s, mainly in association with cardiac catheterizations. With advances in the pharmacological development of contrast media, this type of complication is no longer reported in the literature and thus seems to berare in clinical routine. Thrombembolism induced by catheter manipulation is another possible cause. Lastly, a thrombus that had formed in the distal catheter lumen during the time when the guidewire had been withdrawn into the catheter and the distal lumen was occupied not by the guidewire but by stagnant blood might have been be embolized into the cerebral arteries by injection of contrast medium via the catheter.
Complication Management in Angiography and Interventional Radiology
A complication during angiography is an emergency that requires instantaneous therapeutic reaction. This is especially true for procedurally induced ischemic neurologic deficits. Timely recanalization of the occluded artery is the only effective treatment for acute ischemic stroke. The appropriate management depends entirely on the pathological cause of the complication. An immediate analysis of the clinical situation and the underlying pathology thus is warranted. Also, the radiologist/neuroradiologist must be experienced in the endovascular procedures that are required and must have available all the technical and pharmacological requirements for carrying out the endovascular interventions. For this reason, all international guidelines on the performance of angiography and interventional radiology put high emphasis on the competence of the radiologists/neuroradiologists and the radiology technicians as well as on the quality of the angiographic facilities and equipment, the physiologic monitoring, the resuscitation system, and the procedural care. To the authors’ knowledge there are no official or approved international guidelines on the appropriate management of complications. The further therapeutic approach rather has to follow the guidelines of the underlying pathology. Thus it is possible that different radiologist/neuroradiologists will assess the pathomechanism of an acute complication differently and consequently advise different therapeutic approaches. Moreover, it must be borne in mind that all endovascular techniques discussed below are associated with considerable risks, which must be traded off against the poor prognosis of acute cerebral ischemia of the posterior cerebral vasculature in each individual patient.
In vasospasms, as is often the case following aneurysmal rupture and subarachnoidal hemorrhage, the most frequently used therapeutic techniques aim to achieve arterial vessel dilatation by means of intra-arterial local administration of vessel-dilating substances such as calcium channel blockers (nimodipine, verapamil), by mechanical balloon angioplasty, or by a combination of both approaches. Whereas balloon angioplasty is applicable only in the proximal cerebral arteries, intra-arterial pharmacological dilatation has the advantage of also acting on smaller distal arterial branches and diffuse vasospasms. Calcium antagonists such as nimodipine reduce the influx of calcium in the smooth-muscle cells through blockage of the voltage-operated calcium channels. This may lead to reduced vascular smooth-muscle constriction and a decrease in the release of vasoactive substances from endothelium and platelets. Clinical studies are ongoing on the clinical effectiveness of intra-arterial endovascular therapy and its potential superiority to intravenous medical management for symptomatic cerebral vasospasms.
In acute thrombembolic ischemia, intravenous administration of fibrinolytic substances such as the recombinant tissue plasminogen activator (rt-PA) within 3 hours of symptom onset is the only US FDA-approved treatment. Innovative intravenous pharmacological reperfusion strategies such as the use of novel fibrinolytic agents (tenecteplase, reteplase, desmetolplase, plasmin, microplasmin), glycoprotein (GP) IIb/IIIa antagonists with platelet disaggregating effects (abciximab and tirofiban), and combination therapies potentially improve the efficacy of clot lysis (fibrinolytics and GP IIb/IIIa agents, fibrinolytics and direct thrombin inhibitors), increase the time window for clot lysis (fibrinolytics and neuroprotectants), and reduce the frequency of hemorrhagic complications (fibrinolytics and vasoprotectants). Recently, new mechanical neuroendovascular devices have shown high recanalization rates with acceptable safety in early studies. Multimodal reperfusion therapy including intra-arterial infusion of thrombolytics (mainly rt-PA) and/or anti-platelet agents (tirofiban), mechanical clot disruption and retrieval, and balloon angioplasty with stent placement are emerging endovascular mechanical reperfusion strategies for the management of major acute thrombembolic vessel occlusions. Recent results suggest that the multimodal endovascular approach results in greater chance of both recanalization of the occluded artery and reperfusion of the ischemic tissue than does intravenous therapy.
References and Further Reading
American College of Radiology (ACR). Practice Guideline for the Performance of Cervicocerebral Angiographies in Adults. http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/iv/cervicocerebral_angio.aspx (accessed January 16, 2011)
Berguer R. Vertebrobasilar ischemia: indications, techniques and results of surgical repair. In: Rutherford RB, ed. Vascular Surgery. 5th ed. Philadelphia: WB Saunders; 2000:1823–1837
Biondi A, Ricciardi GK, Louis Puybasset L, et al. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: Preliminary results. AJNR 2004; 25:1067–1076
Bishop N, Rees MR. Idiosyncratic reaction to intracoronary injection of nonionic contrast media. Clin Radiol 1988; 39: 396–397
Cohen JE, Itshayek E, Moskovici S, et al. State-of-the-art reperfusion strategies for acute ischemic stroke. J Clin Neurosci 2011 [Epub ahead of print]. http://www.ncbi.nlm.nih.gov/pubmed/21256755 (accessed January 25, 2011)
Earnest IV F, Forbes G, Sandok BA, et al. Complications of cerebral angiography: prospective assessment of risk. AJR 1984; 142: 247–253
Fleming G, Shanes JG. Left ventriculography induced coronary artery spasm. Clin Cardiol 1984; 7: 560–562
LeVeen RF, Wolf GL, Biery D. Angioplasty-induced vasospasm in rabbit model. Mechanisms and treatment. Invest Radiol 1985; 20: 938–944
Limbruno U, Petronio AS, Amoroso G, et al. The impact of coronary artery disease on the coronary vasomotor response to nonionic contrast media. Circulation 2000; 101: 491–497
Mani RL, Eisenberg RL, McDonald jr EJ, Pollock JA, Mani RJ. Complications of catheter cerebral arteriography: analysis of 5,000 procedures. I. Criteria and incidence. AJR 1978; 131: 861–865
Mani RL, Eisenberg RL. Complications of catheter cerebral arteriography: analysis of 5,000 procedures. III. Assessment of arteries injected, contrast medium used, duration of procedure, and age of patient. AJR 1978; 131: 871–874
Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies. Stroke 2005; 36: 2311–2320
Morris PP. Practical Neuroangiography. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2007
Satoh A, Matsuda Y, Sakai H, et al. Coronary artery spasm during cardiac angiography. Clin Cardiol 1990; 13: 55–58
Tountopoulou A, Ahl B, Weissenborn K, Becker H, Goetz F. Intra-arterial thrombolysis using rt-PA in patients with acute stroke due to vessel occlusion of anterior and/or posterior cerebral circulation. Neuroradiology 2008; 50: 75–83
Williams M, Patil S, Toledo EG, et al. Management of acute ischemic stroke: current status of pharmacological and mechanical endovascular methods. Neurol Res 2009; 31: 807–815
Willinsky RA, Taylor SM, terBrugge K, Farb RI, Tomlinson G, Montanera W. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology 2003; 227: 522–528
History and Clinical Findings
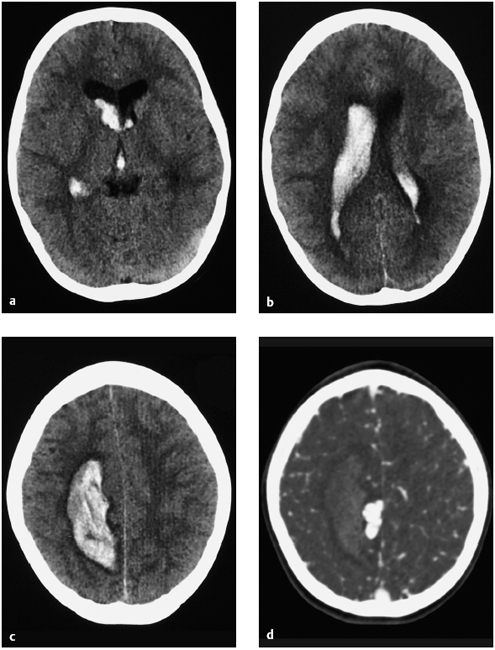
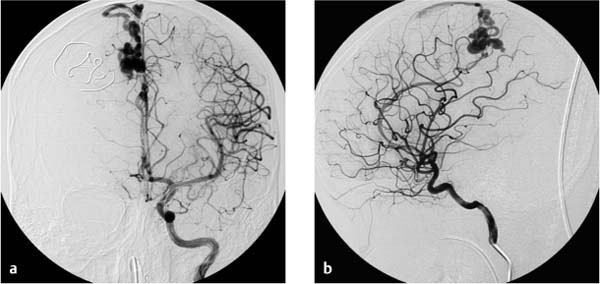
A healthy 9-year-old boy experienced sudden weakness on the left side of his body. Cranial CT revealed a hemorrhage in the right hemisphere with intraventricular rupture caused by a bleeding arteriovenous (AV) malformation (Fig. 7.7). Ventricular dilatation was consistent with grade 1 hydrocephalus due to obstruction of CSF flow by blood clots in the aqueduct. Pressure was relieved by establishing CSF drainage through a right frontal bur hole. Diagnostic angiography of the cerebral arteries confirmed an AV malformation (Fig. 7.8). Since angiography showed only one large-caliber feeder from the right pericallosal artery, it was decided to occlude the AV malformation by endovascular embolization.
Fig. 7.7a–d CT demonstrates a hematoma with intraventricular rupture high in the right parietal white matter (a–c) due to bleeding from an AV malformation (d).
a–c CT scans without contrast medium.
d CT angiography.
Following superselective catheterization of the feeder vessel, a mixture of Ethibloc and lipiodol was injected under fluoroscopic guidance. When the result was checked by angiography, it was found that an estimated 1 mL of the embolic material had passed through the high-flow angioma and into the superior sagittal sinus (Fig. 7.9). At that point the embolization mixture was made more viscous by altering the mixing ratio, and the embolization was continued. The final angiogram showed a smaller per-fused residual nidus.
CT scans the day after the intervention confirmed virtually complete occlusion of the AV malformation (Fig. 7.10).
Fig. 7.8a, b Angiography of the left internal carotid artery shows an AV malformation fed by the right pericallosal artery with a patent anterior communicating artery. Dressing material is projected over the right frontal bone after placement of a CSF drain.
Fig. 7.9a–d Angiography immediately after embolization. Embolic material is visible in the superior sagittal sinus and left sigmoid sinus.
Fig. 7.10a–c Noncontrast CT scans the day after the intervention. The vascular malformation is filled with embolic material (see Fig. 7.7d). A ventricular drain has been placed through a right frontal bur hole. The hematoma appears unchanged relative to earlier images.
Further Case Summary
Two days after the intervention, clinical and laboratory parameters indicated the development of pleuropneumonia (Fig. 7.11) and hepatitis. The patient was moved to the ICU and placed on positive-pressure ventilation. At times his pulmonary status was classified as life-threatening. Chest radiographs showed embolic material projected over both lower lung zones. Based on experience with pulmonary emboli, the degree of embolization of terminal pulmonary artery branches was not considered sufficient to explain the patient’s condition. Since laboratory inflammatory markers were elevated, the embolic injury was obviously accompanied by a toxic component, and the patient was treated accordingly. One week later the patient was transferred from the ICU to general in-patient care. He showed subsequent improvement of left-sided hemiparesis.
Arteriovenous Malformations (AV Angiomas)
An AV malformation (AVM) is an angiodysplasia of the brain with an estimated prevalence and incidence of 1–10/100 000 population per year. Asymptomatic AVMs are probably more prevalent than symptomatic AV angiomas. The latter usually present between 20 and 50 years of age with intracerebral hemorrhage and, less commonly, headaches or seizures. Two-thirds of hemorrhages involve the brain parenchyma. Subarachnoid and intraventricular hemorrhages are less common. The bleeding risk is estimated at 2–4% per year and is increased 2- to 4-fold after a previous bleed. AVMs are characterized by their location as cortical AVMs that drain into the superior sagittal sinus or as deep, central AVMs.
In planning the embolization of an AVM, it is important to identify the transit arteries because they supply the nidus as well as the surrounding healthy brain tissue. Most AVMs have a high arteriovenous shunt volume (high-flow angiomas), but low-flow lesions are also encountered. A high shunt volume may produce a steal effect that diverts blood away from other brain regions into the angioma. This may lead to cerebral ischemia in extreme cases. The actual collection of tangled vessels, called the nidus, consists of enlarged, densely packed capillaries with an abnormal structure. The nidus is the site where arterial blood flows directly into the veins, by-passing normal capillary pathways. The goal of embolization, then, is to permanently occlude the nidus and its arteriovenous connections. It is common to find drainage patterns involving multiple veins.
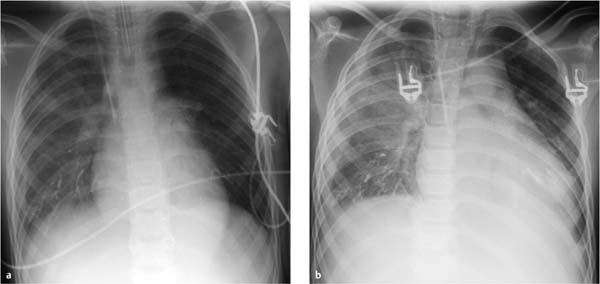
Fig. 7.11a, b Chest radiographs taken during treatment in the ICU for bilateral toxic pleuropneumonia.
a Radiograph 2 days after embolization shows embolic material in both lower lung zones, predominantly on the right side. Other findings are early infiltrates in the right lower lobe and a small pleural effusion on the right side.
b Radiograph 6 days after embolization shows progression of right lung infiltration and the right pleural effusion. New infiltrates have appeared in the left lower lobe. The patient has been extubated.
Error Analysis and Strategy for Error Prevention
The embolic material had passed through the sigmoid sinuses, internal jugular veins, superior vena cava, and right atrium and ventricle and had entered the pulmonary artery. Some of the material had lodged in peripheral branches of the pulmonary artery. The lower lobes were predominantly affected due to the relatively high blood flow in those regions. Toxic inflammation also developed in the lungs and liver.
Anaphylactoid reactions have been described in patients undergoing lymphography with the oily contrast agent lipiodol, an ethyl ester of iodized fatty acids derived from poppy seed oil. Ethibloc, on the other hand, is a bioinert occluding emulsion composed mainly of amidotrizoic acid, zein, oleum papaveris, propylene glycol, and ethanol.
The relatively large diameter of the feeder artery and the high blood flow in the AV malformation led to technical problems during the embolization procedure. Other alternative embolic materials such as starch or Gelfoam particles would also have been carried by the bloodstream across the arteriovenous shunt and into the draining veins and pulmonary circulation. Modern, more advanced embolic agents were not available at the time of the intervention. Open ligation of the pathologic vessels would have been associated with a high surgical risk and a significant risk of potential complications (paralysis).
Endovascular embolization also carries a risk of occluding normal vessels that supply healthy brain parenchyma. Increasing occlusion of the pathologic vessels may cause a redistribution of blood flow, resulting in the undesired embolization of nutrient vessels.
References and Further Reading
Hurst RW, Rosenwasser RH, eds. Interventional Neuroradiology. New York: Informa Healthcare; 2008
Morris PP, ed. Practical Neuroangiography. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2007
History and Clinical Findings
A 55-year-old woman complained of severe headache, dizziness, and nausea of sudden onset. She was verbally responsive when hospitalized but later showed a diminished level of consciousness. She was subsequently sedated, intubated, and placed on mechanical ventilation. Cranial CT revealed a subarachnoid hemorrhage (SAH), an apparent ruptured aneurysm of the left middle cerebral artery (MCA), signs of increased intracranial pressure, and compression of the ventricular system predominantly on the left side (Fig. 7.12). The severity of the SAH was classified as Hunt and Hess grade 4 based on level of consciousness before intubation (see Table 1.3, p. 17).
Fig. 7.12a–d Noncontrast cranial CT scans taken on admission show a predominantly left-sided Fischer grade 4 SAH (see Table 1.4) originating from a perforated aneurysm at the bifurcation of the MCA. Cerebral edema is also present.
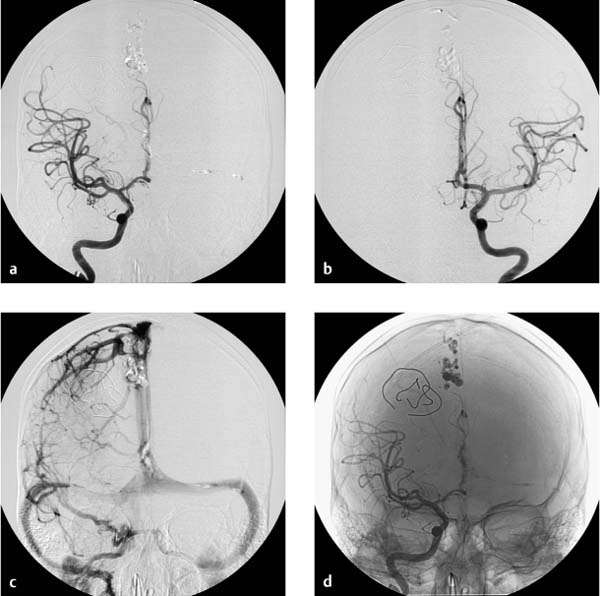
Based on a consultation between neurosurgery and radiology, it was decided to proceed with emergency angiography of the cerebral supply arteries. The principal finding was an aneurysm at the bifurcation of the left MCA, which was ruptured by angiographic criteria and was therefore responsible for the SAH detected by computed tomography (Fig. 7.13). An incidental aneurysm was also noted at the bifurcation of the right MCA, and the terminal segment of the basilar artery was dilated.
Given the severity of the SAH, the frequency of rebleeding during the initial hours after an aneurysm rupture, the configuration of the perforated aneurysm, local vascular topography, and the potential complications and complication rates of endovascular and neurosurgical procedures, it was decided to proceed with endovascular treatment in the same sitting (Fig. 7.14).
A microcatheter was advanced into the left MCA aneurysm through a coaxial catheter system. A 3D coil (length 15 cm, diameter 6 mm) was introduced into the aneurysm lumen under fluoroscopic guidance (Fig. 7.15). Fluoroscopy did not show the marker alignment indicating that the coil was fully deployed and ready for detachment. The peripheral end of the coil extended slightly into the main branch of the MCA. It was then decided to retract and reposition the coil, but the operator could only retract approximately 5 cm of the coil from the aneurysm back into the catheter. Further coil removal was not possible because the loops of the coil had become entangled within the aneurysm lumen. On repeated attempts to pack the platinum coil completely into the aneurysm, the coil broke off near the solder joint with the pusher. At that point, part of the coil was inside the aneurysm, while a straight portion remained within the MCA and the terminal segment of the carotid artery. A retriever system was advanced through the guide catheter and into the internal carotid artery to the site of the coil. Due to the adherent surface of the platinum coil, however, the end of the coil could not be captured with the retrieval snare (Fig. 7.16). These maneuvers were accompanied by a rapidly progressive thrombosis of the left internal carotid artery extending into the MCA. After interdisciplinary consultation, it was decided to schedule the patient for surgical retrieval of the detached and displaced coil and surgical resection of the aneurysm. Postinterventional findings included the detection of spasms and thrombi in the left internal carotid artery, a thrombus at the origin of the left anterior cerebral artery, and an occlusion of the main trunk of the left MCA.
Fig. 7.13a–c Approximately 6-mm aneurysm at the bifurcation of the MCA (a, b). A second aneurysm is noted at an identical site on the right side (c).
a, b Selective angiography of the left internal carotid artery.
c Selective angiography of the right internal carotid artery.
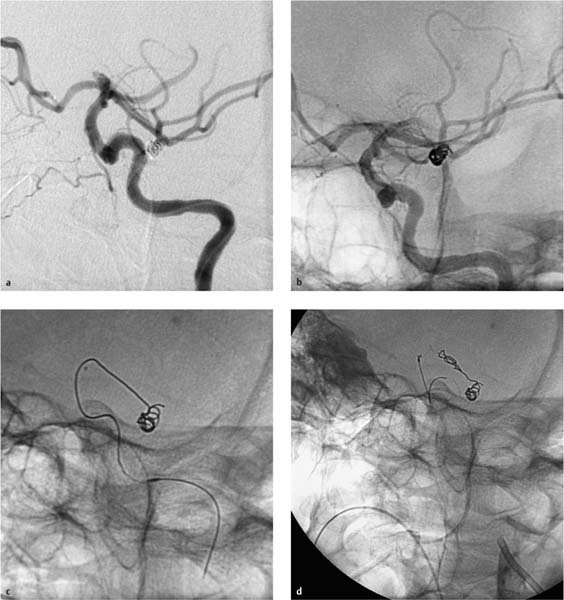
Fig. 7.14a–d Coil occlusion of an aneurysm of the left MCA.
a The platinum coil has been partially deployed into the aneurysm lumen.
b The end of the platinum coil extends slightly into the MCA.
c Incomplete retraction of the coil. The end of the coil lies within the main trunk of the MCA and the terminal segment of the internal carotid artery.
d At the end of the intervention, a large portion of the coil is in the left MCA and induces acute thrombosis.
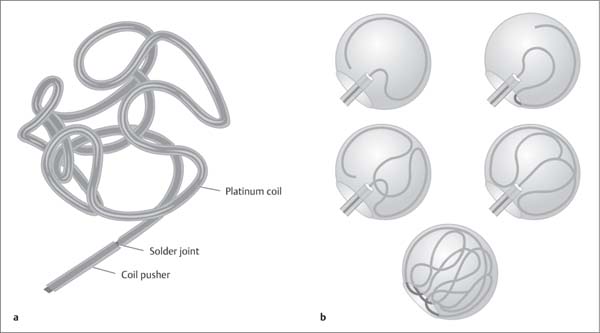
Fig. 7.15a, b Technique of 3D endovascular coiling.
a 3D coil, unraveled.
b Diagrammatic representation of the endovascular occlusion of an aneurysm with a platinum coil.
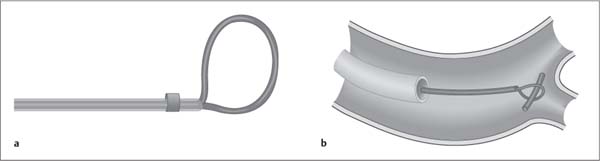
Fig. 7.16a, b Snare principle for the retrieval of an intravascular foreign body.
a Retrieval snare.
b Retrieval of an intravascular foreign body.
Further Case Summary
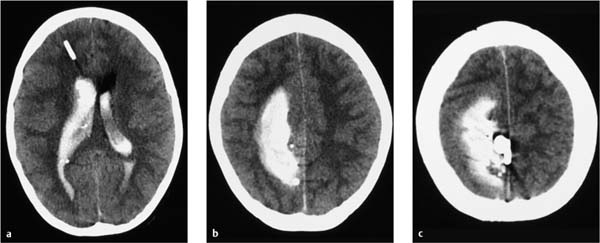
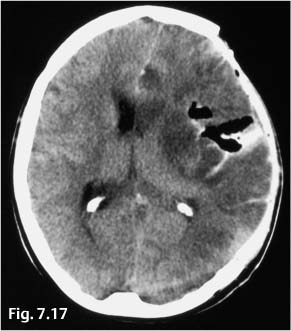
At operation, it was confirmed that the platinum coil was located partly within the aneurysm lumen and partly in the MCA and the terminal segment of the internal carotid artery. There was one site where it had perforated the wall of the aneurysm sac. The coil was retrieved, the aneurysm was resected, the blood clots were removed, and the vessel wall defect was closed with sutures. Transcranial Doppler scans detected blood flow in the main trunk and distal branches of the MCA, confirming that the operation had improved the blood supply to the brain. One day later a hematoma was evacuated and a decompressive craniotomy was performed (Fig. 7.17).
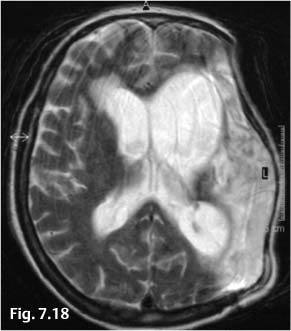
CT follow-up 1 month later showed infarctions in the territories of the left middle and anterior cerebral arteries and edema of the left cerebral hemisphere, which gradually diminished in its intensity and extent. The bone flap was reimplanted 5 months later, and MRI at that time showed a left MCA infarction with compensatory dilatation of the left lateral ventricle (Fig. 7.18).
Fig. 7.17 Noncontrast cranial CT on the first postinterventional day. The hematoma has been evacuated and a decompressive craniectomy has been performed. Increasing ischemia is noted in the left white matter along with predominantly left-sided brain edema.
Fig. 7.18 MRI 5 months after the intervention shows a parenchymal defect and gliosis in the left hemisphere with dilatation of the left lateral ventricle.
Error Analysis and Strategy for Error Prevention
A ruptured aneurysm of the left MCA caused a subarachnoid hemorrhage that was classified as Hunt and Hess grade 4 and Fisher grade 4 (see Table 1.4, p. 18). Diagnostic angiography confirmed the CT suspicion of a ruptured aneurysm at the bifurcation of the left MCA. Based on its location and configuration, the aneurysm was considered accessible to endovascular treatment. Surgical clipping would have been more hazardous due to the brain edema detected by CT and the consequent higher risk of vasospasms.
The complications that arose (coil could not be placed fully within the aneurysm, coil disconnected from pusher wire, failed extraction attempt) have all been described in previously published reports on the endovascular occlusion of ruptured aneurysms with electrolytically detachable platinum coils. Ordinarily, a coil improperly placed in the aneurysm can be retracted into the microcatheter while still attached to the delivery wire. But in this case the coil could not be fully advanced into the aneurysm and could not be withdrawn into the catheter because loops of the coil had become entangled within the aneurysm. As the delivery wire was alternately pulled and pushed in an effort to maneuver the coil into the aneurysm, the solder joint between the pusher and coil was broken. Several possible solutions were available in this situation, but none has been evaluated for safety and efficacy in treatment studies or according to the principles of evidence-based medicine. Thus, the solution applied in any given case is always an individual decision made in an emergency situation, and its efficacy can be evaluated only in retrospect:
 One option is to leave the coil in the parent vessel, followed by anticoagulant therapy with the plasma coagulation inhibitor heparin and/or the platelet aggregation inhibitor acetysalycil acid and/or tirofiban. Since the aneurysm was only partly filled by the platinum coil, this option would have increased the risk of rebleeding. Another problem was that angiography after coil placement showed that thrombi had already formed.
One option is to leave the coil in the parent vessel, followed by anticoagulant therapy with the plasma coagulation inhibitor heparin and/or the platelet aggregation inhibitor acetysalycil acid and/or tirofiban. Since the aneurysm was only partly filled by the platinum coil, this option would have increased the risk of rebleeding. Another problem was that angiography after coil placement showed that thrombi had already formed.
 Another option is to use greater force in an effort to pack the coil into the aneurysm. This maneuver could have caused an additional aneurysm rupture. There was also a risk of dislodging the thrombi (embolic stroke), so this approach was rejected.
Another option is to use greater force in an effort to pack the coil into the aneurysm. This maneuver could have caused an additional aneurysm rupture. There was also a risk of dislodging the thrombi (embolic stroke), so this approach was rejected.
 Endovascular retrieval of the detached coil was tried unsuccessfully at the end of the intervention. More intensive manipulations were withheld to avoid prolonged ischemia since thrombosis had already commenced in the parent vessel.
Endovascular retrieval of the detached coil was tried unsuccessfully at the end of the intervention. More intensive manipulations were withheld to avoid prolonged ischemia since thrombosis had already commenced in the parent vessel.
 Open surgical removal of the intravascular and intraaneurysmal coil was finally performed as a last resort.
Open surgical removal of the intravascular and intraaneurysmal coil was finally performed as a last resort.
Interventional Radiology in the Treatment of Subarachnoid Hemorrhage
Indications
The incidence of SAH is 7–10 per 100 000 population per year. The cause of the hemorrhage in 65–85% of patients is the rupture of an intradural arterial aneurysm. One-third of patients die before reaching the hospital. Another one-third survive with disability despite adequate treatment, and one-third survive without life-altering disability. The peak age incidence is from the fourth through sixth decades.
Rebleeding occurs in approximately 20% of patients during the initial hours after a SAH. Another 20% bleed within the next 2 weeks, and 35% within the first month. The incidence of rebleeding by 6 months after a SAH is greater than 50%. The mortality rate of each rebleed is approximately 50%. The incidence of rerupture thereafter is approximately 3% per year.
The basic treatment goal is to occlude the aneurysm as soon as possible. The main options are surgical clipping and endovascular coiling using an electrolytically detachable platinum coil. Endovascular treatment, introduced clinically in the early 1990 s, has proven superior to the surgical techniques practiced for more than 40 years and has increasingly become the treatment of first choice for aneurysmal SAH. The goal of endovascular treatment is to occlude the aneurysm while maintaining the patency of the parent artery by advancing a platinum coil into the aneurysm through a transfemoral approach. Once deployed within the aneurysm, the coil is electrolytically detached from the delivery wire.
The superiority of endovascular treatment over surgery has been documented by two prospective, randomized two-arm studies that involved 109 patients (Vanninen et al. 1999) and 2143 patients (Molyneux et al. 2002). In the Vanninen study, endovascular treatment had a mortality rate of 1% compared with 4% for surgical treatment. In the Molyneux study, the patients treated by endovascular coiling had such better clinical results than surgically treated patients that the trial was discontinued after the interim evaluation. By 1 year after treatment, 24% of the patients in the endovascular treatment group and 31% of the patients in the surgery group had died or had developed serious disability.
Severity of the SAH as a Prognostic Factor
The severity of the SAH critically affects the prognosis. The causes of a poor outcome after a severe SAH include rebleeding and vasospasms leading to ischemia and increased intracranial pressure with compromise of microcirculation. In a multicenter study by Gallas et al. (2005) involving 650 patients with 705 ruptured aneurysms, the mortality rate was only 1% in patients with a Hunt and Hess grade 1 SAH and 2% in patients with a Fisher score of 1. By contrast, the mortality rates were 22% in patients with a Hunt and Hess score of 4 and 21% in patients with a Fisher score of 4. Weir et al. (2003) reported on 27 consecutive patients that had been treated for a Hunt and Hess grade 4 or 5 aneurysmal SAH by detachable coil embolization. Only 4 of 16 patients with a Hunt and Hess score of 4 (25%) and 3 of 11 patients with a Hunt and Hess score of 5 (27%) had a long-term outcome compatible with a normal life. In 73% and 75% of these cases, respectively, the SAH and subsequent treatment were followed by severe neurologic deficits, an apallic syndrome (7%), or death (59% of the patients died during the first 30 days, 70% within 1 year). Henkes et al. (2004) described a severe neurologic deficit in 27% of 216 patients with a Hunt and Hess grade 4 or 5 SAH. There was a 32% incidence of apallic syndrome or death following coil embolization. In 163 consecutive patients who underwent endovascular treatment of a ruptured intradural aneurysm, Sluzewski et al. (2003) found that a high Hunt and Hess grade of SAH was the most important risk factor for a clinically adverse or fatal outcome (relative risk factor 4.1), followed by technical complications (relative risk factor 3.4), patient age (relative risk factor 1.8), and an aneurysm more than 15 mm in diameter (relative risk factor 1.2).
Complications
To date only a few reports have been published on technical complications during the endovascular treatment of ruptured intracranial aneurysms. These complications have an average reported incidence of 20%. The range from 8% to 63% is explained by small patient groups, different study designs (prospective vs. retrospective, different inclusion criteria), and varying definitions of complications. Coil fragmentation is observed in 1–2% of the procedures. Coil misplacement with protrusion into the parent vessel is reported in 2–6% of cases. The average reported incidence of ischemia (vasospasm, thrombosis, thromboembolism) is 16% (ranging from 4% to 58%).
To the authors’ knowledge, there have been 12 reported cases of coil fragmentation, although the number of unpublished cases is certainly higher (Standard et al. 1994; Kremer et al. 1999; Baltsavias et al. 2000; Raftopoulos et al. 2002; Schumacher and Berlis 2003; Gallas et al. 2005; Schütz et al. 2005). Five fragmentations resulted from an attempt to extract the protruding end of the coil from the parent vessel (Standard et al. 1994; Schumacher and Berlis 2003; Schütz et al. 2005). Raftopoulos et al. (2002) attributed their coil fracture to pressure and shear forces acting on the last coil advanced into an aneurysm lumen that had already been packed with other coils. Four of the 12 fragmentations were successfully treated by endovascular retrieval, resulting in a favorable long-term neurologic outcome in 2 of the 4 patients.
References and Further Reading
Baltsavias GS, Byrne JV, Molyneux AJ, Coley SC, Sohn MJ. Effects of timing of coil embolization after aneurysmal subarachnoid hemorrhage on procedural morbidity and outcomes. Neurosurgery 2000; 47; 1320–1331
Berenstein A, Lasjaunias P, Ter Brugge KG. Surgical Neuroangiography. 2nd ed. Berlin: Springer; 2004
Brilstra EH, Rinkel GJ, Algra A, Van Gijn J. Rebleeding, secondary ischemia, and timing of operation in patients with subarachnoid hemorrhage. Neurology 2000; 55; 1656–1660
Brisman JL, Niimi Y, Song JK, Berenstein A. Aneurysmal rupture during coiling: low incidence and good outcome at a single large-volume center. Neurosurgery 2005; 57: 1103–1109
Cloft HJ, Kallmes DF. Cerebral aneurysm perforations complicating therapy with Guglielmi detachable coils: a meta-analysis. AJNR 2002; 23: 1706–1709
Deng J, Zhao Z, Gao G. Periprocedural complications associated with endovascular embolisation of intracranial ruptured aneurysms with matrix coils. Singapore Med J 2007; 48: 429–433
Fessler RD, Ringer AJ, Qureshi AI, Guterman LR, Hopkins LN. Intracranial stent placement to trap an extruded coil during endovascular aneurysm treatment: technical note. Neuro-surgery 2000; 46: 248–253
Gallas S, Pasco A, Cottier J-P, et al. A multicenter study of 705 ruptured intracranial aneurysms treated with Guglielmi detachable coils. AJNR 2005; 26: 1723–1731
Graves VB, Strother CM, Duff TA, Perl J. Early treatment of ruptured aneurysms with Guglielmi detachable coils: effect on subsequent bleeding. Neurosurgery 1995; 37: 640–647
Henkes H, Fischer S, Weber W, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery 2004; 54: 268–285
Henkes H, Lowens S, Preiss H, et al. A new device for endovascular coil retrieval from intracranial vessels: alligator retrieval device. Am J Neuroradiol 2006; 27: 327–329
Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968; 28: 14–20
Koivisto T, Vanninen R, Hurskainen H, Saari T, Hernesniemi J,Vapalahti M. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke 2000; 31; 2369–2377
Kremer C, Grodon C, Hansen HC, Grzyska U, Zeumer H. Outcome after endovascular treatment of Hunt & Hess grade IV und V aneurysms. Stroke 1999; 30: 2617–2622
Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002; 360; 1267–1274
Raftopoulos Ch, Goffette P, Billa RF, et al. Transvascular coil hooking procedure to retrieve an unraveled Guglielmi detachable coil: technical note. Neurosurgery 2002; 50: 912–915
Schumacher M, Berlis A. The balloon retriever technique. Neuroradiology 2003; 45: 267–269
Schütz A, Solymosi L, Vince GH, Bendszus M. Proximal stent fixation of fractured coil: technical note. Neuroradiology 2005; 47: 874–878
Shin YS, Lee KC, Kim DI, Lee KS, Huh SK. Emergency surgical recanalisation of A1 segment occluded by Guglielmi detachable coil. J Clin Neurosience 2000; 7: 259–262
Sluzewski M, Bosch JA, van Rooij WJ, Nijssen PC, Wijnalda D. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome, and risk factors. J Neurosurg 2001; 94; 238–240
Sluzewski M, van Rooij WJ, Rinkel GJE, Wijnalda D. Endovascular treatment of ruptured intracranial aneurysms with detachable coils: long-term clinical and serial angiographic results. Radiology 2003; 227; 720–724
Standard SC, Tamerla C, Wakhloo AJ, et al. Retrieval of a Guglielmi detachable coil after unraveling and fracture: case report and experimental results. Neurosurgery 1994; 35: 994–999
Thornton J, Dovey Z, Alazzaz A, et al. Surgery following endovascular coiling of intracranial aneurysms. Surg Neurol 2000; 54: 352–360
van Loon J, Waerzeggers Y, Wilms G, Van Calenbergh F, Goffin J, Plets C. Early endovascular treatment of ruptured cerebral aneurysms in patients in very poor neurological condition. Neurosurgery 2002; 50; 457–464
van Rooij WJ, Sluzewski M, Beute GN, Nijssen PC. Procedural complications of coiling of ruptured intracranial aneurysms: incidence and risk factors in a consecutive series of 681 patients. Am J Neuroradiol 2006; 27: 1498–1501
Vanninen R, Koivisto T, Saari T, et al. Ruptured intracranial aneurysms. Acute endovascular treatment with electrolytically detachable coils—a prospective randomized study. Radiology 1999; 211; 325–336
Vora N, Thomas A, Germanwala A, Jovin, T. and Horowitz, M. Retrieval of a displaced detachable coil and intracranial stent with an L5 Merci retriever during endovascular embolization of an intracranial artery. J Neuroimaging 2008; 18: 81–84
Weir RU, Marcellus ML, Do HM, Steinberg GK, Marks MP. Aneurysmal subarachnoid hemorrhage in patients with Hunt and Hess grade 4 or 5: treatment using the Guglielmi detachable coil system. AJNR 2003; 24: 585–590
Zoarski GH, Bear HM, Clouston JC, Ragheb J. Endovascular extraction of malpositioned fibered platinum microcoils from the aneurysm sac during endovascular therapy. Am J Neuroradiol 1997; 18: 691–695
History and Clinical Findings
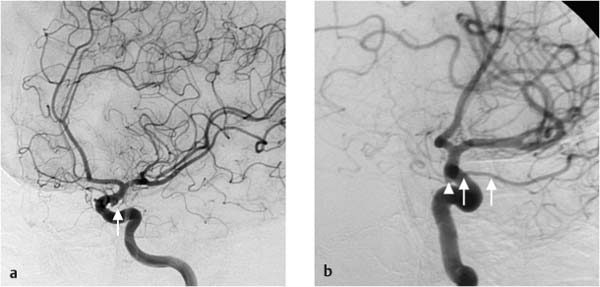
A 52-year-old man with a subarachnoid hemorrhage underwent cerebral angiography (Fig. 7.19). The images showed no abnormalities other than a broad-based protrusion from the inferior surface of the left internal carotid artery in proximity to the posterior communicating artery. The protrusion was interpreted as an aneurysm of the internal carotid artery.
Fig. 7.19a–c Angiography of the left internal carotid artery. The study was described as showing an aneurysm of the internal carotid artery.
Further Case Summary
The next morning a craniotomy was performed to allow surgical clipping of the aneurysm. The exposure revealed a small, funnel-shaped dilatation of the proximal posterior communicating artery, but there was no aneurysm. The source of the subarachnoid hemorrhage was not identified.
Error Analysis and Strategy for Error Prevention
An infundibulum at the origin of the posterior communicating artery (Fig. 7.20) frequently resembles a saccular aneurysm in the terminal segment of the internal carotid artery on angiograms. An infundibulum is defined as a tunnel-or funnel-shaped vascular dilatation caused by the incomplete regression of a fetal artery. The origin of the posterior communicating artery is most commonly affected. Infundibula are occasionally found at the origin of the anterior choroidal artery. The pathognomonic angiographic appearance is that of a rounded or conical expansion of the vessel diameter (up to 3 mm) at its origin from the internal carotid artery, with the distal portion of the posterior communicating artery arising from the center of the dilatation. Infundibular diameters greater than 3 mm have been reported in isolated cases. By contrast, the majority of aneurysms are larger than 3 mm in diameter and are often lobulated. If the distal posterior communicating artery arises eccentrically from a vascular dilatation, an aneurysm should be suspected.
In the case shown, the relationship of the posterior communicating artery to the presumed aneurysm was misinterpreted as a superimposed vessel (Fig. 7.21). This problem may have been solved by taking additional angio-grams at different projection angles to define the vascular relationships more clearly. Rotational angiography would have been an even better option. In this technique the C-arm is rotated 180° around the region of interest during contrast injection and image acquisition, providing a complete cine sequence that allows the vascular territory to be viewed from arbitrary angles in the rotational plane.
In German medical malpractice law, the misinterpretation of angiograms is considered a “diagnostic error.” This denotes an error in the diagnostic interpretation of an imaging procedure. In itself, an objectively wrong diagnosis is not considered to be a treatment error or misinterpretation of findings because radiologists are allowed some leeway in making diagnostic interpretations and decisions. If the interpretation would be considered unreasonable by an expert in the field, the law would define this situation as a simple misinterpretation of findings. If the interpretation is not only unreasonable but is also considered to be an error that a competent physician absolutely would not make and that clearly violates established medical standards, this would justify the assumption of a gross misinterpretation of findings. In the case of a simple misinterpretation, the burden of proof lies with the patient, who must prove that the injury to his or her health would not have occurred, or would have been less severe, if the correct diagnosis had been made. It is often difficult to meet this standard of proof. But if the court decides that a gross treatment error has taken place, the burden of proof lies with the physician.
Fig. 7.20 Infundibular dilatation at the origin of the posterior communicating artery.
Fig. 7.21a, b Relationship of the infundibular origin of the posterior communicating artery (arrowhead in b), which was misinterpreted as a carotid aneurysm, to the distal portion of the posterior communicating artery (arrows).
References and Further Reading
Morris PP, ed. Practical Neuroangiography. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2007
Osborn AG. Diagnostic Cerebral Angiography. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1999
Osborn AG, Blaser SI, Salzmann KL, Katzman GL, Provenzale J, Castillo M. Diagnostic Imaging: Brain. Salt Lake City: Amyrsis; 2004
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree