Cerebral vasospasm (VS) and delayed cerebral ischemia (DCI) are important complications of aneurysmal subarachnoid hemorrhage (ASAH). Imaging approaches to VS monitoring include noninvasive bedside assessment with transcranial Doppler ultrasonography, angiographic evaluation with digital subtraction angiography, and computed tomography (CT) angiography. DCI is a clinical diagnosis and is not fully explained by the presence of angiographic VS. CT perfusion has shown clinical utility and implications for future research in the evaluation of DCI in patients with ASAH. This review article discusses the common approaches to diagnosis and monitoring of VS and DCI, current treatment strategies, and future research directions.
Key points
- •
Angiographic vasospasm (VS) can be monitored with transcranial Doppler ultrasonography; however, computed tomography angiography has a higher diagnostic accuracy when using digital subtraction angiography as the reference standard.
- •
Angiographic VS has limited prognostic value with regard to clinical outcomes in patients with aneurysmal subarachnoid hemorrhage, and delayed cerebral ischemia is not explained by VS alone.
- •
CT-Perfusion can serve as an adjunct modality in the evaluation of DCI given that angiographic VS does not fully explain the clinical syndrome of DCI.
Introduction
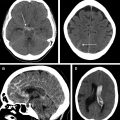
Aneurysmal subarachnoid hemorrhage (ASAH) is a devastating condition with high morbidity and mortality, with a reported incidence ranging from 2.0 to 22.5 per 100,000 depending on the population studied. Approximately 15% mortality and 58% functional disability have been reported in patients surviving the initial aneurysmal rupture, with up to 46% developing long-term cognitive impairment. , Cerebral vasospasm (VS) and delayed cerebral ischemia (DCI) are common serious complications of ASAH, typically occurring between 4 and 14 days after the initial aneurysmal rupture. Understanding of the cause of cerebral hypoperfusion and ischemia after ASAH has evolved over time, with several terms emerging to describe the imaging and clinical features. Thus, angiographic VS is defined as imaging evidence of arterial narrowing on transcranial Doppler ultrasonography (TCD), digital subtraction angiography (DSA), or computed tomography angiography (CTA). Time-of-flight magnetic resonance angiography (MRA) can also be used to evaluate angiographic VS; an early study evaluating anterior circulation VS with MRA found high specificity (95%–99%) for all evaluated vessels, as well as excellent sensitivity for the anterior cerebral artery (ACA) (100%), but low sensitivity for the internal carotid artery (ICA) (25%) and middle cerebral artery (MCA) (56%). DSA has the advantage of allowing immediate treatment with intra-arterial administration of vasodilators and/or balloon angioplasty; limitations include its invasive nature and potential associated complications.
DCI in the setting of ASAH has a more comprehensive definition as a new unexplained focal neurologic deficit (not present at the time of initial admission) or a decrease by 2 points or greater on the Glasgow Coma Scale, and/or new imaging evidence of infarction not attributable to initial aneurysmal rupture. VS and DCI are closely intertwined, because arterial narrowing may result in reduced cerebral blood flow (CBF), which in turn can lead to cerebral hypoperfusion and ischemia. Thus, VS and DCI have been used interchangeably in clinical practice. However, it is important to differentiate the terms because not all patients with imaging evidence of arterial narrowing in VS develop DCI. Furthermore, up to 50% of patients with DCI do not show imaging evidence of angiographic VS. DCI has emerged as the most clinically relevant diagnosis given the strong association with poor clinical outcomes, cognitive impairment, and quality-of-life metrics. DCI is currently diagnosed using a combination of clinical examination, TCD, and CTA/computed tomography perfusion (CTP). , In addition, magnetic resonance (MR) imaging–based studies have shown an added benefit in the evaluation of DCI. Cerebrospinal fluid (CSF) biomarkers may also have potential to aid in diagnosis and prognosis of patients with VS and DCI.
Overview of imaging techniques
In the clinical setting, patients with ASAH are primarily evaluated by clinical examination and TCD. Limitations of clinical examination arise from the variable nature of symptoms as well as the fact that patients with ASAH are intubated and sedated with depressed consciousness. TCD is noninvasive and can be performed at bedside, but it has lower specificity compared with other modalities and is reliant on serial assessments. DSA is the reference standard for detection of angiographic VS and allows administration of therapy in real time; however, it is invasive and associated with periprocedural complications. CTA/CTP has reported high sensitivity and specificity for DCI given the ability to show both angiographic VS as well as perfusion deficits. , MR-based approaches to VS and DCI include DWI to assess for acute infarction, MRA to assess for angiographic VS, arterial spin labeling (ASL) and DCE perfusion to assess for DCI, and vessel wall imaging to assess for inflammation.
Transcranial Doppler Ultrasonography
TCD is a noninvasive technique allowing sensitive assessment of the cerebral vessels for VS and can be performed at the bedside without requiring the use of intravenous contrast or exposing the patient to ionizing radiation. TCD uses the Doppler effect, which allows the blood flow velocity and direction in a given vessel to be calculated based on the difference in sound wave frequency emitted by the transducer and reflected off moving red blood cells back to the transducer at a higher (positive Doppler shift, indicating flow toward the transducer) or lower (negative Doppler shift, indicating flow away from the transducer) frequency. Its ready availability, low cost, and minimal contraindications and complications have made TCD a mainstay of longitudinal VS monitoring of patients with ASAH in the intensive care unit. Limitations of TCD include its lower sensitivity compared with DSA, computed tomography (CT)–based, and MR-based modalities, operator dependence, and challenges related to the quality of the temporal insonation window.
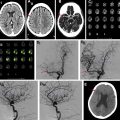
TCD imaging is typically performed with a 5-MHz to 1-MHz sector array transducer. Three acoustic windows are commonly used: transtemporal (allowing visualization of the MCA, ACA, posterior cerebral artery [PCA], and terminal ICA), transforaminal (allowing visualization of the vertebrobasilar system), and transorbital (allowing visualization of the ophthalmic artery and cavernous segment of the ICA). Vessels are evaluated using gray-scale and color Doppler flow imaging. Spectral waveforms are recorded from the bilateral proximal, mid, and distal MCA. Single measurements are obtained in the bilateral ACA, PCA, and ICA terminus. Flow velocity in the extracranial distal ICA is also recorded. Based on the Bernoulli principle, which dictates an increase in flow velocity in a narrowed blood vessel compared with nonstenosed adjacent vessels, mean flow velocity (MFV) can be calculated for each vessel in the circle of Willis ( Fig. 1 ). Diagnostic MFV thresholds for VS are 120 to 150 cm/s (mild), 150 to 200 cm/s (moderate), and greater than 200 cm/s (severe). For the ACA and PCA, diagnostic thresholds for VS are 80 cm/s and 85 cm/s, respectively.

Digital Subtraction Angiography
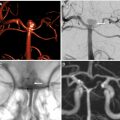
DSA is considered the reference standard for detection and monitoring of angiographic VS. DSA uses real-time fluoroscopic imaging combined with selective catheterization and direct intra-arterial administration of iodinated contrast. DSA allows accurate quantitative assessment of VS severity in each proximal and distal segment of the anterior and posterior circulation. Furthermore, DSA allows immediate treatment of VS through therapeutic endovascular intervention. Two approaches to angioplasty have been described and used separately or in combination: balloon angioplasty and chemical angioplasty (also known as intra-arterial vasodilator infusion therapy). Drawbacks of DSA include its invasive nature with associated risk for iatrogenic complications, its limited availability given the need for an experienced operator and an angiography suite equipped with appropriate staff (ie, technologist and nurse), as well as associated radiation exposure.
DSA is performed by obtaining intra-arterial access and placing a catheter, typically into the superficial femoral artery. The catheter is then navigated into the great vessels under fluoroscopic guidance and maintained in the cervical common carotid artery or cervical ICA. Iodinated contrast is injected to visualize vascular anatomy, and serial anteroposterior and lateral images are obtained. Typically, the bilateral carotid arteries and at least 1 vertebral artery are catheterized. If VS is diagnosed, treatment can be administered in the form of intra-arterial vasodilators (chemical angioplasty) or balloon angioplasty, and repeat imaging is performed to assess for treatment response in real time. The risks associated with DSA include vessel wall injury including rupture, thrombosis and embolism, stroke, bleeding, infection, hematoma, and renal dysfunction.
Computed Tomography Angiography
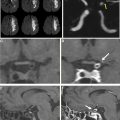
CTA of the head is a widely available imaging technique that can be quickly performed without the need for specialized equipment or processing software. Early studies and a subsequent meta-analysis showed high sensitivity and specificity of CTA for detection of angiographic VS with either DSA or clinical DCI as the reference standard. Helical CTA is based on rapid scanning after bolus injection of iodinated contrast, and allows assessment of vessel caliber in the circle of Willis. New scanning protocols, such as dynamic shuttle technique, allow reduction in radiation dose and iodinated contrast. In addition, CTP can be performed in conjunction with CTA; however, there may be some limitations with regard to coverage, particularly in the posterior fossa. Overall, the noninvasive nature of CTA allows serial scanning of patients at risk or those showing early signs and symptoms of VS and/or DCI.
CTA is commonly performed on a 64-section CT scanner using 45 to 90 mL of iohexol, followed by a 30-mL to 45-mL saline bolus. Typical scanning parameters include 120 kV tube voltage, 250 mAs, pitch of 0.6, section thickness of 0.625 mm from C2 to the vertex, field of view of 30 to 32 cm, and matrix size of 512 × 512. Postprocessing reformations include 1-mm axial and 3-mm coronal and sagittal reformats. Three-dimensional reformations of the anterior and posterior circulation are additionally generated.
Computed Tomography Perfusion
CTP is based on continuously acquired CT images obtained during the wash-in and wash-out phases of an intravenous bolus of iodinated contrast. Dedicated postprocessing software is needed to generate parametric maps for subsequent clinical interpretation. CTP parameters commonly used in clinical practice are mean transit time (MTT), CBF, and cerebral blood volume (CBV). In particular, MTT and CBF have been found to have the greatest discrimination ability for the presence of DCI. , In addition, CTP with extended-pass technique has been shown to have promise in the evaluation of blood-brain barrier (BBB) dysfunction using permeability surface area product (PS) parametric maps, which may predict the development of subsequent DCI. Given the dissociation of angiographic VS with clinical DCI and with subsequent poor clinical outcomes, CTP is increasingly being used to evaluate cerebral hypoperfusion as the key pathophysiologic mechanism in VS/DCI.
CTP is commonly performed along with noncontrast CT and CTA as part of our routine clinical care protocol in patients with ASAH. Noncontrast CT is performed using the following parameters: 120 kVp, 250 mAs, 1.0 rotation time, and 5.0 mm collimation. CTP scanning is performed with axial shuttle mode protocol for simultaneous acquisition of CTA and CTP data using the following parameters: 80 kVp, 400 mAs, 0.4 rotation time, 5.0 mm collimation with 17 cine cycles and 2.8-second interscan delay for the first pass. Second-pass technique includes 10 cine cycles with a 10-second interscan delay. A total of 45 mL of nonionic contrast is intravenously administered at 4.0 mL/s followed by a 30-mL saline-bolus chaser.
An arterial input function (AIF) and venous output function (VOF) are selected (placed automatically with most current software packages) and parametric maps are generated based on the time-density curve for each voxel.
Magnetic Resonance Imaging
MR-based imaging is well established for the evaluation of acute infarction using diffusion-weighted imaging (DWI). Advantages of MR include the high contrast resolution and lack of ionizing radiation. MR imaging in patients with ASAH has traditionally been regarded to be of lower clinical utility given its longer acquisition time, higher cost, and individual patient contraindications to MR imaging (eg, severe claustrophobia, allergy to gadolinium-based contrast, or presence of MR-incompatible hardware). However, recent advances in MR-based vascular imaging, particularly vessel wall imaging (VWI), as well as perfusion imaging with ASL and dynamic contrast-enhanced (DCE) techniques, have challenged this view.
There is currently no standardized protocol for MR-based monitoring of VS and DCI in patients with ASAH. DWI has utility in the acute setting to evaluate for acute infarction. ASL and DCE perfusion–based assessment of cerebral perfusion to assess for DCI has been proposed in the literature. , VWI showed correlation between vessel wall enhancement and DCI/poor clinical outcomes.
Imaging findings
There are 2 main approaches to imaging VS: imaging arterial luminal narrowing (angiographic VS) and imaging microvascular dysfunction of the brain parenchyma (DCI). Although angiographic VS has historically been the focus of imaging, evaluating DCI has increasingly gained importance because it has been shown to be more closely associated with clinical outcomes.
Vasospasm
Transcranial Doppler findings of vasospasm
The Bernoulli principle dictates that the flow velocity in a narrowed blood vessel is increased compared with nonstenosed adjacent vessels. From the spectral Doppler waveform, MFV can be calculated for each vessel in the circle of Willis, and MFV thresholds for TCD-based diagnosis of VS have been determined. Importantly, MFV increase alone is not sufficient to diagnose VS given that flow velocity can increase with physiologic conditions such as hyperemia. The Lindegaard ratio aims to correct for this by concurrently measuring the MFV in the MCA and the ipsilateral extracranial ICA (EICA). A Lindegaard ratio (MFV MCA/MFV EICA) of 3 to 6 indicates mild to moderate VS, and a ratio greater than 6.0 indicates severe VS (see Fig. 1 ). An increased MFV with a normal Lindegaard ratio suggests hyperemia or other non-VS causes of increased flow velocity. This approach is less well validated for the posterior circulation. Of note, a later study found higher than previously indicated diagnostic accuracy with very high sensitivity (90%) and negative predictive value (92%), supporting the role of TCD as a noninvasive screening tool at the bedside.
Digital subtraction angiography findings of vasospasm
There is no unified grading scale for angiographic VS on DSA. Typically, qualitative assessment applied to each proximal and distal segment of the circle of Willis stratifies the degree of VS based on the degree of luminal narrowing as mild (<25% narrowing), moderate (25%–50% narrowing), and severe (>50% narrowing). A recent retrospective study in 45 patients found angiographic VS to predominantly involve the anterior circulation. In concordance with other previous reports, there was no significant correlation between degree of angiographic VS and clinical outcomes.
Computed tomography angiography findings of vasospasm
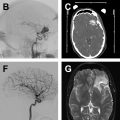
There is no unified grading scale for angiographic VS on CTA. Similar to the approach to DSA described earlier, qualitative assessment applied to each proximal and distal segment of the circle of Willis stratifies the degree of VS based on degree of luminal narrowing, analogous to the DSA-based assessment ( Fig. 2 ). Semiquantitative grading scales incorporating a point system applied to each vascular segment have been proposed.